ORIGINAL RESEARCH ARTICLE
An alternative to opioid-based intravenous patient controlled analgesia in severe burn patients undergoing full thickness split graft in upper limbs
Bo-Fu Shiha, Fu-Yu Huanga, Shih-Jyun Shena,e, Chih-Wen Zhenga, Chao-Wei Leeb,c, Ming-Wen Yanga, An-Hsun Choua,b, Shiow-Shuh Chuangb,d, Hsin-I Tsaia,b
aDepartment of Anesthesiology, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan; bCollege of Medicine, Chang Gung University, Taoyuan, Taiwan; cDepartment of General Surgery, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan; dDepartment of Plastic Surgery, Burn Unit, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan; eDepartment of Mechanical Engineering, Chang Gung University, Taoyuan, Taiwan
ABSTRACT
Background: Opioids provide good analgesic effect in burn patients during acute phase, but these patients may develop tolerance after prolonged exposure. Alternative analgesic strategies such as peripheral nerve blocks appear to provide adequate pain control while sparing opioid-related side effects. The purpose of this study was to evaluate intravenous patient-controlled analgesia (IV-PCA) and continuous peripheral nerve block (CPNB-PCA) in severe burn patients with relatively young age undergoing repeated debridement and large-area full thickness skin graft (FTSG).
Methods: The records of victims in dust explosion in Taiwan in 2016 from Chang Gung Memorial Hospital Pain Service Database between 2016 June and 2017 December were evaluated. The patients’ demographic data including age, gender, weight, burn area, degree of burn, type of PCA regimen (IV-PCA versus CPNB-PCA), size of FTSG, and adverse effects were collected.
Results: The total in-hospital morphine consumption was significantly lower in CPNB-PCA than IV-PCA group. A trend of decrease in numerical rating scores (NRS) was observed for both groups and CPNB group had comparable NRS than IV-PCA group at rest. On movement, CPNB grouped had significantly lower NRS than IV-PCA on post-operative day 3.
Conclusion: Our study demonstrated that in patients requiring high dosage of opioid, CPNB may be a suitable alternative for pain control.
KEYWORDS: Continuous peripheral nerve block; patient controlled analgesia; full thickness split graft; burn
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 56–61. DOI: https://doi.org/10.2340/jphs.v58.12292.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 9 April 2023; Accepted: 5 June 2023. Published: 1 August 2023.
CONTACT Hsin-I Tsai tsaic@hotmail.com, Department of Anesthesiology, Chang Gung Memorial Hospital, Linkou Medical Center, No.5, Fuxing St., Guishan Dist., Taoyuan City 33305, Taiwan (R.O.C.).
Competing interests and funding: The authors have no conflicts of interests to declare.
Background
For patients with severe burn injuries, defined as second degree or more burn injuries involving at least 20% of a patient’s total body surface area, usually require initial resuscitative measures followed by intensive care unit management. These patients may also require repeated and aggressive wound debridement to remove non-viable tissues followed by burn wound closure and later reconstructive surgery for severe contractures or disfiguring scars [1]. With such frequent surgical procedures, post-operative pain management can become a challenge for the intensivists/physicians. As the perception of pain can be quite variable among burn patients, efforts should be made to provide adequate pain relief. Well controlled pain is associated with better wound healing, sleep, recovery and quality of life [2]. Without effective pain control, aside from the debilitating experience secondary to acute pain, other long-term morbidities such as chronic pain, allodynia, post-traumatic stress, anxiety, depression and even suicidal ideation may develop [3–6]. To manage burn pain pharmacologically, paradigm-based treatment approaches for burn pain through five different phases of injury, treatment and recovery has been described, including background, procedural, breakthrough, post-operative and chronic pain. Background pain is present when the patient is at rest, with low to moderate intensity and long duration. Opioids are often the first line of choice in pharmacologic pain relief and continuous intravenous (IV) opioid infusion such as patient controlled analgesia (PCA) is often utilized for background pain. The effectiveness of analgesia for repeated wound care such as dressing changes is of paramount importance as anticipatory anxiety may develop when adequate analgesia is not provided for an initial, painful procedure [7]. Breakthrough pain occurs when opioid requirements are increased secondary to changes in the burn wound that increase in pain, inadequate background analgesic management or development of opioid tolerance that require a change in the pharmacologic management when post-operative pain may require the use of continuous regional block and additional opioid dosing [8]. Opioids appear to provide good pain control in acute phase; however, patients may develop opioid tolerance and opioid-induced hyperalgesia after prolonged exposure [9]. Chronic pain, on the other hand, is the result of damages sustained by the nerve endings in the skin and is often of neuropathic character, best managed with opioid and non-opioid analgesics [10]. Measures should be taken to prevent opioid escalation in long-term opioid therapy.
In recent years, peripheral nerve blocks (PNB) have been widely used in patients receiving upper limb surgery for the management of post-operative acute pain. The use of regional nerve blocks may provide adequate pain relief while sparing opioid-related side effects. Nerve blocks of upper extremity can be divided into four parts: interscalene, supraclavicular, infraclavicular and axillary [11]. While considering surgical site below shoulder, supraclavicular, infraclavicular and axillary nerve blocks are commonly used. All three approaches provide adequate quality of surgical anesthesia when guided by ultrasound and comparable post-operative analgesia duration [11,12]. Infraclavicular brachial plexus block may be preferred to the supraclavicular approach when complications, such as Horner’s syndrome, dyspnea, and pneumothorax [13] are taken into consideration. Furthermore, continuous infusion of local anesthetics via an infraclavicular catheter provides superior post-operative analgesic effect in comparison to supraclavicular catheter [14].
A few years ago, a devastating color dust explosion happened in Taiwan, leading to 15 deaths and 484 severe burn injuries. The Ministry of Health and Welfare of Taiwan initiated the casualty management system, and 49 burn patients were sent to a tertiary hospital for further care. After the initial resuscitative care, the survivors were supported in the intensive care unit, followed by repeated wound debridement. Most of the victims were previously healthy young adults without history of illicit drug use. During the hospitalization, all patients initially received intravenous patient-controlled analgesia (IV-PCA) constituted of opioids for acute pain management. These patients underwent excision of necrotic tissue in the burn area to a depth until viable bleeding tissue was identified. Patients were discharged from the hospital when burn wound was closed and became stable. A few months after the initial burn injury, these patients would subsequently receive reconstructive surgeries to improve the aesthetic and function status. In this study, our aim is to determine, in severe burn patients who require regular pain medication, whether opioid-based regimen remains the optimal pain management modality in acute post-surgical pain control. We have compared patient controlled continuous peripheral nerve blocks (CPNB-PCA) with IV-PCA in severe burn patients following reconstructive surgery with large-area full thickness skin graft (FTSG) at our hospital.
Methods
Patient selection
Under the approval of Institutional Review Board of Chang Gung Memorial Hospital (CGMH) (IRB 202001774B0), we acquired data from CGMH Pain Service Database. All methods were performed in accordance with the relevant guidelines and regulations in accordance with the Declaration of Helsinki. Informed consents were waivered by the CGMH IRB. This study enrolled the victims of the previously described dust explosion in Taiwan in 2016 and received FTSG reconstructive surgery in the upper limbs in CGMH between 2016 June and 2017 December. These patients received either IV-PCA or CPNB-PCA as post-operative pain management. The patients’ demographic data including age, gender, weight, the size (percentage of burn to total body surface area) and initial degree of burn, type of PCA regimen (IV vs. CPNB) and size of FTSG were collected. Patients who did not use PCA, whose FTSG involved more than one arm and who had a history of neuropathy at the surgical site or previous history of illicit drug use were excluded from the study. Patients were allocated into one of the two groups according to the type of analgesic modalities chosen at the time of surgery: IV-PCA (n = 17), CPNB-PCA (n = 39).
Intraoperative procedure
All patients received a standardized general anesthetic technique for induction with IV 50–100 mcg fentanyl, 2–3 mg/kg propofol and 0.2 mg/kg cisatracurium and 20–40 mg lidocaine hydrochloride. Maintenance was achieved with oxygen/air sevoflurane and IV fentanyl. For those who would receive CPNB-PCA, a catheter under ultrasound guidance was placed in the infraclavicular brachial plexus region and secured after general anesthesia. Tourniquet bands were applied to the upper arm for FTSG. The donor skin was harvested, trimmed to adequate thickness and applied to the recipient site. The FTSG was sutured and tied, and the donor site was closed. Upon completion of the surgery, all patients received IV-PCA or CPNB-PCA upon arriving burn unit.
Post-operative analgesics protocol
The content of the IV-PCA bag was comprised of 500 µg fentanyl and 40mg morphine with normal saline to a total of 340 mL, giving an equivalent morphine concentration of 0.27 mg/mL, whereas CPNB bag was constituted with 600 mg bupivacaine and 300 µg fentanyl with normal saline to a total of 600 mL, giving 1 mg/mL of bupivacaine and 0.5 μg/mL of fentanyl in the CPNB mixture. IV-PCA was initially set up with a basal infusion dose at 2 mL/h, bolus dose of 4 mL, lockout time of 5 min and 4-h limit of 50 mL while CPNB-PCA was initially set up with a basal infusion of 5 mL/h, bolus dose of 5 mL, lockout time of 20 min and 4-h limit of 50 mL. The regimens may be adjusted according to the level of pain by the responsible acute pain service practitioner at discretion. All patients were given instructions on how to use PCA and followed up for the next three post-operative days. At the burn units, additional opioids could be administered in both groups if the intensity of pain was recognized as equal to or more than moderate on a verbal severity scale (no pain, mild, moderate, severe and extreme pain). For the next 3 days, the acute pain service personnel would record the cumulative consumption of PCA and the presence of opioid-related side effects in IV-PCA group, the presence of regional local anesthetic related side effects in CPNB-PCA group and pain intensity based on the numeric rating scale (NRS) at rest and upon movement in both groups. The NRS is an 11-point numeric scale, with 0 representing no pain, and 10 representing the worst pain imaginable. For the convenience of comparison of opioid requirement, the total amount of opioids consumed on the ward over a 3-day period was converted to their equivalent morphine dose using the equianalgesic conversion ratios of fentanyl: morphine = 0.1:10 [15].
Statistical analysis
The basic characteristics of the patients were analyzed by Chi-Square test and Fisher’s test. The possible side effects of opioids and local anesthetics were listed and analyzed by Student’s-t test. The NRS of patients at rest and on movement were similarly analyzed. A p < 0.05 was defined as statistically significant. All statistical data were analyzed by the SPSS statistical software.
Results
Of the victims of dust explosion, there were 200 patients receiving hand surgery from 2016 June to 2017 December. After excluding the patients who met the exclusion criteria, 56 patients were included in the study, among which, 17 received IV-PCA and 39 selected CPNB-PCA as shown in Figure 1. In Table 1, these patients had an average age of 21.77 ± 2.91 and 22.92 ± 4.08 years and body weight was 60.29 ± 12.74 and 67.25 ± 14.04 kilograms (kg) in IV-PCA and CPNB-PCA groups, respectively. Most patients had an initial burn injury covering 50–69% of body surface area (BSA). Among these burn victims, 3rd degree burns more than 69% BSA were less common. The average harvested area of FTSG was 176.47 ± 83.81 and 229.69 ± 141.74 cm2 in IV-PCA and CPNB-PCA group, respectively, with no statistical difference. However, CPNB-PCA group required much less morphine (21.82 ± 8.47 vs. 96.53 ± 59.17 mg) when compared with IV-PCA group during the first three post-operative days. The pre-operative and post-operative photographs of burn wound were shown in Figure 2.
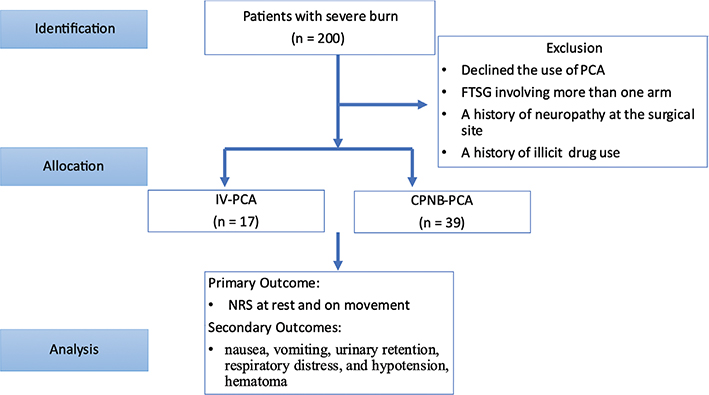
Figure 1. Flowchart in patient identification, allocation and analysis.
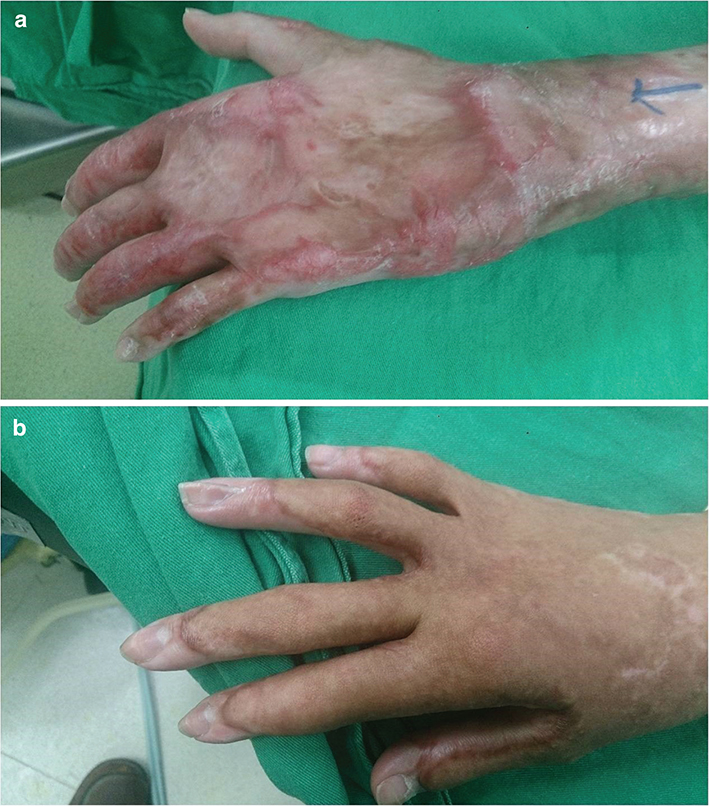
Figure 2. Severe burn before large area FTSG (a) and after FTSG (b).
Primary outcome
NRS at rest and on movement in the first three post-operative days were collected. As shown in Table 2, the NRS were compared on a daily basis (day 1 vs. day 2 and day 2 vs. day 3). In IV-PCA and CPNB-PCA group, the improvement in analgesic effect was significant only from post-operative day 1 to day 2 at rest, but not on movement. On the other hand, CPNB-PCA group showed a significant decrease in pain score on movement from day 2 to day 3. In Table 3, NRS at rest and on movement were compared between the two groups. CPNB-PCA group showed comparable analgesic effects to IV-PCA group both at rest and on movement on the first three post-operative days except for day 3 on movement. As shown in Table 3 and Figure 3, a trend of a decrease in pain was observed for both groups and CPNB-PCA group had comparable or lower NRS than IV-PCA group at rest and on movement during the first three post-operative days.
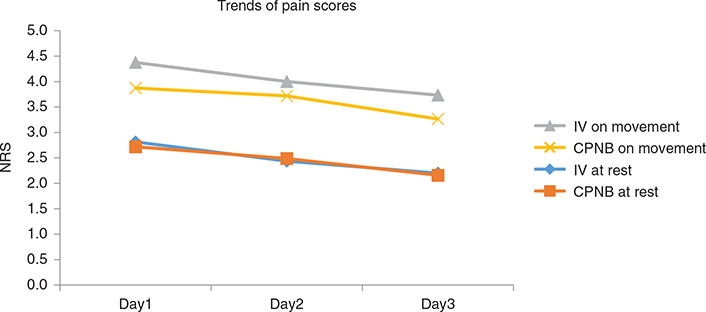
Figure 3. Pain scores at rest and on movement using IV-PCA or CPNB.
NRS, numerical rating score; IV, intravenous; PCA, patient controlled analgesia; CPNB, continuous peripheral nerve block.
Secondary outcomes
Adverse effects related to IV-PCA and CPNB-PCA were presented in Table 4. No difference in nausea, vomiting, urinary retention, respiratory distress, and hypotension was observed in the two groups. No nerve injury was documented for the CPNB group and was not demonstrated in Table 4. On average, IV-PCA group had a drop of 8.35 ± 18.72 mmHg while CPNB had 12.08 ± 12.41 mmHg in systolic blood pressure, but no statistical difference was observed. Although post-operative hematoma of the FTSG site was observed in some of the patients, no statistical difference in the incidence was detected in the two groups.
Discussion
Burn patients can experience a variable level of pain intensity with a long recovery. Pain, with definition as an unpleasant sensory and emotional experience associated with acute or potential tissue damage from International Association for the Study of Pain, is a response related to a range of noxious stimuli including heat, cold, and mechanical. For patients in acute phase of burn injury, severe tissue damage and cytokine-related inflammatory response may lead to moderate to severe pain, increasing the difficulty in adequate pain management [10]. The management of burn pain is complex as pain caused by burn injury itself as well as the pain related to therapeutic procedure or operation need to be cared for delicately. In addition, pain caused by burn has been described as long-lasting in association with severe psychological disturbances under inappropriate pain control [16]. Literature has shown that burn patients may require education on pain itself and alternative treatments in addition to pain medications and their associated addiction risk [17]. In this study, we have attempted to examine the efficacy of an alternative pain control modality to IV opioid in severe burn patients who have undergone multiple surgeries from initial wound debridement to subsequent reconstructive surgeries.
For most conditions, morphine is widely used for pain management in patients with burn injury. However, some well recognized side effects related to opioid such as nausea, constipation, and respiratory depression should be taken into consideration when high dosage is required for pain relief. Other detrimental effects of prolonged opioid exposure includes tolerance, dependence, hyperalgesia, and addiction, all of which may lead to increased opioid requirement without the anticipated analgesic effect [18]. The use of multimodal analgesia incorporating non-opioid component may add analgesic effects via other pain signaling pathway, reduce adverse effects and reduce opioid requirement. Adjunctive therapies for pain management in burn patients encompass pharmacologic and non-pharmacological therapies. Recent literature has revealed that virtual reality is a distraction intervention to relieve pain and distress [19,20]. A decrease in pain score with variable effects on opioid requirement especially in the management of background pain and procedural pain has been observed [21]. Unfortunately, limited options for neuropathic pain and no non-opioid adjuncts are available for breakthrough pain.
Local anesthetics, widely used in regional anesthesia and analgesia, are often useful for post-operative pain control, especially in combination with other analgesics in multimodal analgesia protocol. The practicality of PNB to facilitate early active mobilization and functional recovery after hand surgery related to adequate pain control has already been demonstrated [22,23] and PNB in single shot as well as continuous infusion format both provided adequate pain control for children after reconstructive surgery for burn wound and offered satisfying result for the skin graft [24]. PNB appears to be a suitable alternative to opioid when complications such as neurologic injury may be minimized when blocks are performed under ultrasound guidance [25, 26]. Therefore, PNB should be considered as a suitable alternative for pain management, but one has to bear in mind that it is not without risks.
Our study has shown that CPNB lowered the pain level in severely burnt patients who required reconstructive surgery with large-area FTSG without increasing the risks of adverse effects. However, small population size and a lack of randomization secondary to the nature of retrospective studies are some of the limitations to the study. As the majority of the patients were young adults naïve to opioid use, the generalizability of our study may also be limited to patients who are opioid dependent. Further prospective studies recruiting a larger population size, including patients who require repeated operations and analgesics during their hospital stay are required. That said, we have successfully demonstrated that in patients requiring regular pain medication, CPNB may still be a suitable, opioid-sparing alternative for adequate pain management.
Conclusion
In severe burn patients, well controlled pain is associated with better wound healing, sleep, recovery and quality of life. We revealed that CPNB-PCA showed comparable analgesic effect as IV-PCA with a reduction in opioid requirement in such patients admitted for repeated debridement or skin-grafting surgery of upper limbs.
Acknowledgments
The study was approved by the Institutional Review Board of CGMH (IRB 202001774B0); Consent to participate was waived due to the retrospective nature of the study.
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
References
[1] Zuo KJ, Medina A, Tredget EE. Important developments in burn care. Plast Reconstruct Surg. 2017;139(1):120e–138e. https://doi.org/10.1097/prs.0000000000002908
[2] Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13(5–6):337–346. https://doi.org/10.1159/000104862
[3] Saxe GN, Stoddard F, Hall E, et al. Pathways to PTSD, part I: children with burns. Am J Psychiatry. 2005;162(7):1299–1304. https://doi.org/10.1176/appi.ajp.162.7.1299
[4] Brown NJ, Kimble RM, Gramotnev G, Rodger S, Cuttle L. Predictors of re-epithelialization in pediatric burn. BurnsJ Int Soc Burn Inj. 2014;40(4):751–758. https://doi.org/10.1016/j.burns.2013.09.027
[5] Edwards RR, Magyar-Russell G, Thombs B, et al. Acute pain at discharge from hospitalization is a prospective predictor of long-term suicidal ideation after burn injury. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S36–S42. https://doi.org/10.1016/j.apmr.2007.05.031
[6] Yuxiang L, Lingjun Z, Lu T, et al. Burn patients’ experience of pain management: a qualitative study. Burns. 2012;38(2):180–186. https://doi.org/10.1016/j.burns.2011.09.006
[7] Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med. 1998;152(2):147–149. https://doi.org/10.1001/archpedi.152.2.147
[8] Cuignet O, Mbuyamba J, Pirson J. The long-term analgesic efficacy of a single-shot fascia iliaca compartment block in burn patients undergoing skin-grafting procedures. J Burn Care Rehabil. 2005;26(5):409–415. https://doi.org/10.1097/01.bcr.0000176885.63719.7e
[9] Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. 2019;33(10):943–955. https://doi.org/10.1007/s40263-019-00660-0
[10] Griggs C, Goverman J, Bittner EA, Levi B. Sedation and pain management in burn patients. Clin Plast Surg. 2017;44(3):535–540. https://doi.org/10.1016/j.cps.2017.02.026
[11] Brattwall M, Jildenstål P, Stomberg M, Jakobsson J. Upper extremity nerve block: how can benefit, duration, and safety be improved? An update. F1000Research. 2016;5:907. https://doi.org/10.12688/f1000research.7292.1
[12] Tarıkçı Kılıç E, Akdemir MS. Comparison of supraclavicular, infraclavicular, and axillary approaches for ultrasound-guided brachial plexus block for upper limb surgeries: a rretrospective analysis of 182 blocks. Dubai Med J. 2018;1(1–4):33–37. https://doi.org/10.1159/000496235
[13] Koscielniak-Nielsen ZJ, Frederiksen BS, Rasmussen H, Hesselbjerg L. A comparison of ultrasound-guided supraclavicular and infraclavicular blocks for upper extremity surgery. Acta Anaesthesiol Scand. 2009;53(5):620–626. https://doi.org/10.1111/j.1399-6576.2009.01909.x
[14] Mariano ER, Sandhu NS, Loland VJ, et al. A randomized comparison of infraclavicular and supraclavicular continuous peripheral nerve blocks for postoperative analgesia. Regional Anesth Pain Med. 2011;36(1):26–31. https://doi.org/10.1097/AAP.0b013e318203069b
[15] Dinges HC, Otto S, Stay DK, et al. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Anal. 2019;129(4):1153–1162. https://doi.org/10.1213/ane.0000000000003887
[16] Latarjet J, Choinère M. Pain in burn patients. Burns J Int Soc Burn Inj. 1995;21(5):344–348. https://doi.org/10.1016/0305-4179(95)00003-8
[17] Duchin ER, Moore M, Carrougher GJ, et al. Burn patients’ pain experiences and perceptions. Burns. 2021;47(7):1627–1634. https://doi.org/10.1016/j.burns.2021.01.010
[18] Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet (London, England). 2019;393(10180):1558–1568. https://doi.org/10.1016/s0140-6736(19)30430-1
[19] Indovina P, Barone D, Gallo L, Chirico A, De Pietro G, Giordano A. Virtual reality as a distraction intervention to relieve pain and distress during medical procedures: a comprehensive literature review. Clin J Pain. 2018;34(9):858–877. https://doi.org/10.1097/ajp.0000000000000599
[20] Hoffman HG, Boe DA, Rombokas E, et al. Virtual reality hand therapy: a new tool for nonopioid analgesia for acute procedural pain, hand rehabilitation, and VR embodiment therapy for phantom limb pain. J Hand Ther. 2020;33(2):254–262. https://doi.org/10.1016/j.jht.2020.04.001
[21] Kim DE, Pruskowski KA, Ainsworth CR, Linsenbardt HR, Rizzo JA, Cancio LC. A review of adjunctive therapies for burn injury pain during the opioid crisis. J Burn Care Res. 2019;40(6):983–995. https://doi.org/10.1093/jbcr/irz111%
[22] Otsuka T, Okamoto H, Mizutani J, Goto H, Sekiya I. Continuous peripheral nerve blocks for early active mobilization after hand surgery: four case reports. J Hand Surg Asian-Pacific. 2018;23(3):419–423. https://doi.org/10.1142/s2424835518720281
[23] Mehrotra S, Dua A, Singh V, Mehare S, Kaundal R. The role of regional block analgesia in the early functional recovery of burns in the hand. Case Report. 2017;25(1):85–87. https://doi.org/10.4103/ijb.ijb_25_17
[24] Shank ES, Martyn JA, Donelan MB, Perrone A, Firth PG, Driscoll DN. Ultrasound-guided regional anesthesia for pediatric burn reconstructive surgery: a prospective study. J Burn Care Res. 2016;37(3):e213–e217. https://doi.org/10.1097/bcr.0000000000000174
[25] Mellecker C, Albright J, Clark R. Peripheral nerve blocks and incidence of post-operative neurogenic complaints and pain scores. Iowa Orthopaed J. 2012;32:83–89.
[26] Jeng CL, Torrillo TM, Rosenblatt MA. Complications of peripheral nerve blocks. Br J Anaesth. 2010;105(Suppl 1):i97–107. https://doi.org/10.1093/bja/aeq273