ORIGINAL RESEARCH ARTICLE
Size adjustment suture technique for lymphaticovenular anastomosis
Satoshi Onoda, PhD; Kahori Tsukura, MD and Toshihiko Satake, PhD
Department of Plastic and Reconstructive, Aesthetic Surgery, Toyama University Hospital, Toyama, Japan
Abstract
In this report, we describe a super microsurgical technique that enables rapid and accurate anastomosis while adjusting for caliber differences when anastomosing a small-caliber lymphatic vessel and a vein with a larger caliber, which is frequently encountered in surgeries such as lymphaticovenous anastomosis (LVA).
The suture size adjustment technique was performed in 30 anastomoses of lymphatic vessels and veins, whose diameter of lymph duct was at least two times smaller than that of the vein. The type of lymphedema, caliber of lymphatic vessels and veins anastomosed, caliber ratio, vein wall thickness, modified caliber ratio after vein wall thickness subtracted, presence of additional anastomosis, and anastomosis time were examined. On average, the lymphatic vessels had a diameter of 0.61 mm, while the veins were 1.43 mm in diameter. The mean caliber ratio of vein to lymphatic vessel was 2.3, while the modified caliber ratio of vein-to-lymphatic vessel was 1.5 on average. The average venous wall thickness was 0.51. The average anastomosis time was 9.1 min and no additional anastomosis due to leakage was necessary in any case.
We successfully performed an anastomosis of lymphatic vessels and veins with different calibers, which can maintain long-term patency while adjusting the caliber difference and suppressing leakage at the anastomosis site. Finally, the caliber of the vein is commonly larger than that of the lymphatic vessel to be anastomosed in many cases of LVA surgery, indicating that the proposed anastomosis method could be of therapeutic use in many cases.
KEYWORDS: Lymphaticovenous anastomosis; super-microsurgery; caliber difference; long-term patency; obstruction
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 155–158. DOI: https://doi.org/10.2340/jphs.v58.18384.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 9 August 2023; Accepted: 23 October 2023; Published: 29 December 2023.
CONTACT Satoshi Onoda satoshiprs18@gmail.com Department of Plastic and Reconstructive, Aesthetic Surgery, Toyama University Hospital, 2630 Sugitani, Toyama, Toyama 930–0194, Japan
Introduction
Surgical treatment of lymphedema includes lymphaticovenous anastomosis (LVA) [1–6], vascularized lymph node transfer [7–9], and liposuction [10–12]. These surgical indications and procedures are generally determined according to various factors such as the stage of edema, patient general condition, and implementation of self-care [13]. LVA is indicated for patients from the early to late stages of lymphedema because of its preventive effect against cellulitis [14], in addition to edema reduction. Moreover, LVA is minimally invasive and can be performed under local anesthesia [15], in the elderly [16], and prophylactically [17]. Previously, we conducted experimental studies in a rat model and reported several key points for maintaining long-term patency after LVA and factors that need to be considered during anastomosis [18, 19]. More specifically, since the lymphatic vessels targeted by LVA are very small (< 1 mm in most cases) and have extremely thin walls, anastomosis requires specific precautions and more advanced techniques than that of conventional microsurgery that targets blood vessels of 2 or 3 mm in diameter [20–22].
In LVA, it is necessary to anastomose the neighboring lymphatic vessels and veins, which often inevitably results in an anastomosis with a difference in caliber between the two [23, 24]. In this report, we aimed to describe a super microsurgical anastomosis technique that enables an accurate anastomosis in a short time, while adjusting for caliber differences when anastomosing a small-caliber lymphatic vessel and a vein with a larger caliber, which is frequently encountered in LVA [25].
Materials and methods
This study was performed under a protocol approved by the Toyama University Hospital ethics committee. From June 2021 to December 2022, the suture size adjustment technique (SSAT) was performed in 30 anastomoses of lymphatic vessels and veins, whose diameter of lymph duct was at least two times smaller than that of the vein and difficult to anastomose using the conventional anastomosis method.
The type of lymphedema, caliber of anastomosed lymphatic vessels and veins, caliber ratio, vein wall thickness, modified caliber ratio after vein wall thickness subtracted, thread size, presence of venous reflux requiring clipping, presence of additional anastomosis by leak, and anastomosis time were examined. For various measurements, a crack scale was used (Figure 1).
Figure 1. Crack scale used to evaluate clinical parameters of the anastomoses. This scale enables accurate measurement of the length in 0.5 mm increments.
SSAT procedure
The veins and lymphatic vessels suitable for anastomosis were identified through skin incision. The criteria for selecting the veins to be anastomosed were as follows: small caliber difference from the lymphatic vessel to be anastomosed, tension-free placement during anastomosis, and little to no back bleeding from the cut end [21]. Another crucial aspect was the presence of a high lymphatic fluid flow in the lymph vessels. Otherwise, the lymphatic vessels with minimal degeneration and fibrosis were selected. The final judgment regarding flow was based on the findings.
The procedure was started by cleaning and trimming connective and fatty tissue around the lymphatic vessels and veins. The lymphatic vessels and veins were then placed in a tension-free position and anastomosis initiated. Firstly, a stay suture was performed on both sides. We aimed to minimize the suture width in the lymphatic vessel wall so that it was not teared. For the vein wall, the needle was inserted from the edge of the end of the intima, and at the outer membrane, and placed approximately 0.2 to 0.5 mm away (same as the thickness of the vein wall) from the edge of the end of the vein. Following the execution of the stay suture on both sides, the end of the lymphatic vessel wall and the vein wall were in a state in which the intimas were grounded, while the outer membrane of the vein covered the end of the lymphatic vessel. Subsequently, the anterior wall was anastomosed using the same needle entry and exit points as those used in the stay suture. A trumpet-like spreading of the lymphatic vessel wall was achieved after the stay sutures, making it easier to puncture the needle in its anterior and posterior walls. The vascular canal was then reversed, and the posterior and anterior walls were anastomosed in the same manner, with the use of two to three needles each. Since the lymphatic vessel wall is stretched like a trumpet into the vein, leakage from the anastomosis is unlikely to occur (Video 1). Finally, the anastomotic vessels were placed in a tension-free position, and the wound was closed without twisting or compression.
Results
The targeted lymphedema included three primary and 27 secondary anastomoses. The average diameter of the lymphatic vessels was 0.58 mm (range, 0.40 to 1.60 mm), while the veins were 1.40 mm (range, 0.70 to 3.80 mm). Moreover, the veins were larger in diameter than the anastomosed lymph vessels in all cases, while the mean caliber ratio of vein-to-lymphatic vessel was 2.4 (range, 2.0 to 3.2). The average venous wall thickness (×2) was 0.48 mm (range, 0.2 to 1.2 mm), and the modified caliber ratio of vein to lymphatic vessel was 1.55 on average (range, 1.2 to 2.0). The thread thickness used was 11-0 nylon in all patients. Venous reflux was observed in two anastomoses; however, no additional anastomosis due to leakage from the anastomosis was required in any case. The average anastomosis time was 9.17 min (range, 7 to 16 min) (Table1).
Discussion
In LVA, the lymphatic vessels and veins must be identified and anastomosed within few centimeters of the skin incision wound. Although the upper and lower extremities had some differences in dimensions, most of the targeted lymphatic vessels ranged from 0.5 to 0.7 mm in diameter, indicating a lack of opportunities to anastomose lymphatic vessels with a diameter of ≥ 1 mm. In comparison, several veins had a diameter of ≥1 mm, underscoring that a certain degree of difference in caliber are inevitable in the majority of anastomoses. In addition to caliber differences, the walls of the lymphatic vessels and veins varied in thickness: walls of the lymphatic vessels were generally < 0.1 mm, while those of veins approximately 0.2–0.3 mm. Furthermore, cases with even thicker walls can be observed in instances of wall thickening due to inflammation. According to our previous investigation of LVA, when the subintimal tissue is exposed at the anastomotic site, platelets from the resulting influx of blood may aggregate and induce thrombus formation, which can be a major factor in early occlusion [19]. In addition, leakage of lymphatic fluid and blood from the anastomotic site can induce skin lymphatic leakage and hematoma formation around the anastomotic lymphatic vessels, and cause lymphatic and venous compression. Therefore, the ideal LVA is the one in which lymphatic and venous intima are in close contact (intima-to-intima) at the anastomosis site and no leakage occurs from the anastomosis site. However, some ingenuity is required to achieve an ideal anastomosis as the one described above in the case of lymphatic vessels and veins that have large caliber differences between them and different wall thicknesses.
SSAT advantages
The bite of the anastomosis should be sufficiently minimal to prevent tearing of the wall with respect to the lymphatic vessels. An excessively large bite during anastomosis leads to twisting of the anastomosis site and exposure of the subintimal tissue. Regarding the vein wall, the bite should be equal to the thickness of the vein wall. In addition, the needle is delivered at an angle from the end of the venous intima toward the outer membrane (Figure 2). This procedure creates a pinpoint of contact between the lymphatic vessel wall and the intima. Furthermore, the shapes of the lymphatic vessels and veins at the anastomosis are conformed to one another by making maximum use of the extensibility of the lymphatic vessels. Finally, the small-caliber lymphatic vessel wall is trumpeted in the direction of the vein, facilitating subsequent needle insertion. Our technique has several clinical advantages. Firstly, the submucosal tissue of the vein is not exposed at the anastomotic site and occlusion of the anastomotic site difficult to occur. Secondly, the cross-sectional surfaces of the vein and lymphatic vessels at the anastomotic part are matched, reducing the likelihood of leakage from the anastomotic site. Lastly, this procedure facilitates the insertion of the needle into the wall after stay suture and shortens the anastomosis time. Alternatively, if lymphatic vessels and veins are sutured with similar bites without using the SSAT method, lymphatic vessels with a small caliber are entrapped in the vein, resulting in an incorrectly shaped cross-sectional surface of vein, preventing accurate anastomosis and increasing the chance of leaks from the anastomosis point (Figure 3).
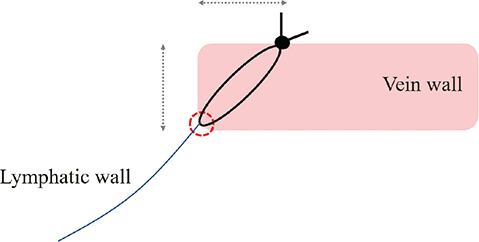
Figure 2. Junction of lymphatic vessels and veins. A contact point (red circle) between the lymphatic vessel and the vein is formed at the end of the vein’s intima. The needle penetrates the vein wall at an angle. Bite of the vein should be as thick as the thickness to the vein (gray arrows).
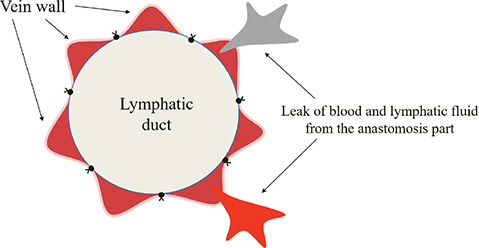
Figure 3. Undesirable anastomotic configurations. The vein ends are bumpy, and the lymphatic and venous ends do not adhere to each other.
Adjustment of caliber difference
In SSAT, the lymphatic vessel wall and the intimal portion of the vein wall are sutured to adjust the caliber difference between the lymphatic vessel and vein while anastomosing. Hence, the lymphatic vessel is anastomosed with the same lumen minus as the vein minus its thickness. Specifically, when a lymphatic vessel with a diameter of 0.60 mm and a vein with a diameter of 1.20 mm and a wall thickness of 0.20 mm are anastomosed, the vein wall is two times larger than the lymphatic vessel wall in terms of the diameter ratio. However, when SSAT is used to suture the lymphatic wall and the intimal portion of the vein, the actual vein diameter of the anastomosis is 0.8 mm after subtracting the vein wall thickness on both sides. In this case, the ratio becomes 0.6/0.8 = 1.33, which facilitates the adjustment of the diameter difference during anastomosis. Regarding the stitches, minimizing their number is desirable to prevent leakage at the anastomosis site. Therefore, the usual anastomosis is sutured using six to eight stitches (Figure 4).
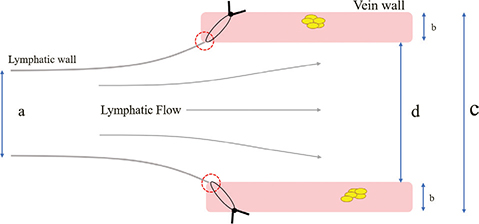
Figure 4. Schematic diagram of the size adjustment suture technique (SSAT). (a) Diameter of the lymph vessels. (b) Thickness of the vein wall. (c) Diameter of the vein. (d) Diameter of the vein after SSAT correction. Red circle: Intimal junction of lymphatic vessels and veins. The SSAT allows to reduce the true diameter of the vein and corrects the difference in caliber between the lymphatic vessels and veins. Additionally, it prevents leakage from the suture by drawing the lymphatic vessel’s disconnection in the direction of the vein wall.
Limitation
The LVA is a delicate surgical technique that requires a high degree of skill, which may have technical implications for the result of this article.
Conclusion
In the current study, we have successfully performed an anastomosis of lymphatic vessels and veins with different calibers, which can maintain patency while adjusting the caliber difference and suppressing leakage at the anastomosis site. Since the caliber of the vein is commonly larger than that of the lymphatic vessel to be anastomosed in many cases of LVA surgery, the proposed anastomosis method could be of therapeutic use in many cases.
References
[1] Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010 Sep; 126(3): 752–758. https://doi.org/10.1097/PRS.0b013e3181e5f6a9
[2] Olszewski WL. Lymphovenous microsurgical shunts in treatment of lymphedema of lower limbs: a 45-year experience of one surgeon/one center. Eur J Vasc Endovasc Surg. 2013 Mar; 45(3): 282–290. https://doi.org/10.1016/j.ejvs.2012.11.025
[3] Campisi C, Boccardo F, Zilli A, et al. Long-term results after lymphatic-venous anastomoses for the treatment of obstructive lymphedema. Microsurgery. 2001; 21: 135–139. https://doi.org/10.1002/micr.1025
[4] Onoda S, Masahito K. The utility of the airborne technique for lymphaticovenular anastomosis. Plast Reconstr Surg. 2019 Feb; 143(2): 459e–460e. https://doi.org/10.1097/PRS.0000000000005251
[5] Onoda S, Komagoe S. Lymphaticovenular anastomosis for Klippel–Trenaunay–Weber syndrome. Int J Surg Case Rep. 2019; 58: 67–69. https://doi.org/10.1016/j.ijscr.2019.04.023
[6] Onoda S, Kinoshita M. Involved stich technique for super-microsurgical anastomosis. J Plast Reconstr Aesthet Surg. 2020 Jun; 73(6): 1174–1205. https://doi.org/10.1016/j.bjps.2020.02.013
[7] Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol. 2015 Sep; 22(9): 2919–2924. https://doi.org/10.1245/s10434-015-4408-4
[8] Hunter CL, Chang DW. Discussion: anatomical basis of the gastroepiploic vascularized lymph node transfer: a radiographic evaluation using computed tomographic angiography. Plast Reconstr Surg. 2018 Oct; 142(4): 1053–1054. https://doi.org/10.1097/PRS.0000000000004796
[9] Roka-Palkovits J, Steinbacher J, Tinhofer I, et al. Treatment of cervicofacial lymphatico-venous malformation with vascularized lymph node transfer (VLNT). Plast Reconstr Surg. 2018 Sept; 142(3): 425e–426e. https://doi.org/10.1097/PRS.0000000000004628
[10] Brorson H, Ohlin K, Olsson G, et al. Quality of life following liposuction and conservative treatment of arm lymphedema. Lymphology. 2006 Mar; 39(1): 8–25.
[11] Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg. 2016 Jan; 32(1): 56–65. https://doi.org/10.1055/s-0035-1549158
[12] Brorson H. From lymph to fat: liposuction as a treatment for complete reduction of lymphedema. Int J Low Extrem Wounds. 2012 Mar; 11(1): 10–19.
[13] Onoda S, Nishimon K. The utility of surgical and conservative combination therapy for advanced stage lymphedema. J Vasc Surg Venous Lymphat Disord. 2021 Jan; 9(1): 234–241. https://doi.org/10.1016/j.jvsv.2020.05.007
[14] Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009 Mar; 16(3): 703–708. https://doi.org/10.1245/s10434-008-0270-y
[15] Todokoro T, Furniss D, Oda K, et al. Effective treatment of pelvic lymphocele by lymphaticovenular anastomosis. Gynecol Oncol. 2013 Feb; 128(2): 209–214. https://doi.org/10.1016/j.ygyno.2012.11.014
[16] Onoda S, Kinoshita M. Lymphaticovenular anastomosis in elderly patients. J Plast Reconstr Aesthet Surg. 2020 Jun; 73(6): 1174–1205. https://doi.org/10.1016/j.bjps.2020.02.022
[17] Onoda S, Todokoro T, Hara H, et al. Minimally invasive multiple lymphaticovenular anastomosis at the ankle for the prevention of lower leg lymphedema. Microsurgery. 2014 Jul; 34(5): 372–376. https://doi.org/10.1002/micr.22204
[18] Onoda S, Kimata Y, Matsumoto K. A novel lymphaticovenular anastomosis rat model. Ann Plast Surg. 2016 Mar; 76(3): 332–335. https://doi.org/10.1097/SAP.0000000000000571
[19] Onoda S, Kimata Y, Matsumoto K, et al. Histologic evaluation of lymphaticovenular anastomosis outcomes in the rat experimental model: comparison of cases with patency and obstruction. Plast Reconstr Surg. 2016 Jan; 137(1): 83e–91e. https://doi.org/10.1097/PRS.0000000000001884
[20] Onoda S, Kimata Y, Sugiyama N, et al. Analysis of 10-year training results of medical students using the Microvascular Research Center Training Program. J Reconstr Microsurg. 2016 Jun; 32(5): 336–341. https://doi.org/10.1055/s-0035-1568884
[21] Onoda S, Kimata Y, Goto A. The drop-down technique as an optimal technique for back-wall end-to-side anastomosis. J Craniofac Surg. 2014 Jul; 25(4): 1435–1437. https://doi.org/10.1097/SCS.0000000000000754
[22] Onoda S, Sakuraba M, Asano T, et al. Thoracoacromial vessels as recipients for head and neck reconstruction and cause of vascular complications. Microsurgery. 2011 Nov; 31(8): 628–631. https://doi.org/10.1002/micr.20947
[23] Visconti G, Salgarello M, Hayashi A. The recipient venule in supermicrosurgical lymphaticovenular anastomosis: flow dynamic classification and correlation with surgical outcomes. J Reconstr Microsurg. 2018 Oct; 34(8): 581–589. https://doi.org/10.1055/s-0038-1649518
[24] Hayashi A, Visconti G. Supermicrosurgical lymphaticovenular anastomosis: a practical textbook. Morrisville, North Carolina, United States: lulu.com. 2020 HV Editions.
[25] Onoda S, Kimata Y, Matsumoto K. Iliolumbar vein as a training model for microsurgical end-to-side anastomosis. J Craniofac Surg. 2016 May; 27(3): 767–768. https://doi.org/10.1097/SCS.0000000000002501