ORIGINAL RESEARCH ARTICLE
Autologous fat transplantation prior to permanent expander implant breast reconstruction enhances the outcome after two years: a randomized controlled trial
Anna Lindegrena,b, Inkeri Schultzc,d, Åsa Edsander-Nordc,d, Jacinth Yane and Marie Wickmand,f
aDepartment of Breast Surgery, Södersjukhuset, Stockholm, Sweden; bDepartment of Clinical Science and Education, Karolinska Institute Södersjukhuset, Stockholm, Sweden; cDepartment of Plastic- and Craniofacial Surgery, Karolinska University Hospital, Stockholm, Sweden; dDepartment of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, Sweden; eStatistician, Karolinska Institute, Institute of Environmental Medicine, Stockholm, Sweden; fSophiahemmet Hospital, Stockholm, Sweden
ABSTRACT
Radiotherapy is important in breast cancer treatment. A side effect of the treatment is fibrosis that decreases the possibility for a successful breast reconstruction with expanders and with high patient satisfaction with the result. The most common option for mastectomized, irradiated women wishing for a breast reconstruction is autologous tissue transplantation. However, some patients are not suitable for flap surgery. Fifty mastectomized and irradiated women were included in a randomized controlled trial. They underwent breast reconstruction with expanders and were allocated 1:1 to either receive pre-treatment with autologous fat transplantation (AFT) or not. Primary outcomes were frequency of reoperations and complications. Secondary outcomes were number of days in hospital, number of outpatient visits to surgeon or nurse and patient reported outcome as reported with Breast Q. Follow-up time was 2 years. Fifty-two per cent of the intervention group and 68% of the controls underwent reoperations (p = 0.611). Thirty-two per cent of the intervention group and 52% of the controls had complications (p = 0.347). The median number of consultations with the nurse was four in the intervention group and six in the control group (p = 0.002). The AFT patients were significantly more satisfied with their breasts and psychosocial well-being after 2 years. They also had higher increase in satisfaction with breasts, psychosocial well-being, and sexual well-being when comparing baseline with 2 years postoperatively. This randomized controlled trial indicates benefits of AFT prior to breast reconstruction with expanders, especially on patient reported outcome even if the study sample is small.
KEYWORDS: Breast reconstruction; radiotherapy; autologous fat transplantation; lipofilling; fat grafting; mastectomy; expander implants; patient reported outcome; complications; reoperations
Citation: Journal of Plastic Surgery and Hand Surgery 2024; 59: 65–71. DOI: https://doi.org/10.2340/jphs.v59.18622.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 1 September 2023; Accepted: 30 January 2024; Published: 20 May 2024.
CONTACT Anna Lindegren anna.lindegren@ki.se Department of Breast Surgery, Södersjukhuset, Stockholm; Department of Clinical Science and Education, Karolinska Institute Södersjukhuset, Sjukhusbacken 10, SE-11883 Stockholm, Sweden
Competing interests and funding: The authors report that there are no competing interests to declare. The funding bodies took no part in any of the stages in the research.
Introduction
Radiotherapy is essential in breast cancer treatment. It reduces recurrences and breast cancer specific mortality [1, 2]. Unfortunately, adverse effects of radiotherapy are a well-known problem, even if improved irradiation programs have decreased the unwanted side effects. A common and not as feared as damages on heart and lungs [3], is radiodermatitis, which often emerges within weeks after radiotherapy but may also develop after several years. Clinical presentations of radio dermatitis include atrophy and fibrosis of the skin and underlying tissue [4–6]. Radio dermatitis also increases the complication rate if breast reconstruction is performed [7, 8]. Breast edema, unevenness, and capsular contracture around the breast implant are common problems [9–11] leading to morbidity, deteriorated aesthetic result, pain and unwanted reoperations [11, 12]. Complete failure of the breast reconstruction with implant loss is more common in patients after treatment with radiotherapy [7, 12]. Thus, women who have undergone mastectomy and radiotherapy are generally not recommended reconstruction with implants but autologous tissue transplantation, preferably without implants. The latissimus dorsi flap has been widely used, but the deep inferior epigastric perforator flap has become more popular as it does not require implants to achieve desired volume. However, some patients are not suitable for flap surgery some patients are not interested in flap surgery, even though it would be ideal for them and opt for expander reconstruction if feasible. The outcome after implant-based breast reconstruction varies from excellent to inferior aesthetic result with capsular contracture and harder breast than desired because of the fibrosis in the tissues surrounding the implant. Reoperation rate for delayed breast reconstruction after radiotherapy was at the time of study design not reported in the literature but in our experience up to 80% (all indications). Visible rippling of the implant, skin indurations, asymmetry and bad scar healing is common, problems that can be treated with autologous fat transplantation (AFT) to the area. Clinical studies have shown that AFT improves the quality of irradiated tissue and seems to reverse radio dermatitis [13–15]. It has also been shown that gene expression alterations related to radio dermatitis can be normalized with AFT [16]. Salgarello et al. [15, 17] prepared the chest wall with AFT in mastectomized, irradiated women before implant-based breast reconstruction. After this treatment they reported no complications and high patient reported outcome (PRO) using the BREAST-Q questionnaire [18]. Case-series [19–21] correspondingly report good results with this method. No experimental studies have confirmed this. We aimed to investigate if AFT before expander reconstruction can decrease complications and reoperations, compared to expander reconstruction alone by performing a randomized controlled trial. We also wanted to assess PRO compared to the control group. The hypothesis is that AFT to the irradiated tissue prior to expander surgery leads to less morbidity, better aesthetic outcome and patient satisfaction. If this would be the case, expander reconstruction after mastectomy and radiotherapy could be an alternative for more patients if AFT is added to the treatment protocol.
Materials and methods
This randomized controlled trial has an allocation ratio of 1:1. Inclusion criteria were mastectomized women who had had radiotherapy, aged 25–70 years and not suitable or opting for breast reconstruction with flaps. At the first breast reconstruction surgery at least 1 year should have passed since previous breast surgery or radiotherapy, and a radiological examination not older than 3-month, to rule out cancer recurrences, was required. Exclusion criteria were no current local recurrence or distant metastases, contraindication for anesthesia, severe systemic disease and BMI above 30. Two senior plastic surgeons treated all patients. Data were collected from the patients’ medical records. The tax-funded Swedish health insurance covered all treatment costs.
The patients in the intervention group underwent AFT with injections into the pectoralis major muscle as well as in the subcutaneous fat of the chest 70–180 days before expander surgery with the aim to transplant a minimum of 100 cc fat to the reconstruction site in one or more sessions. AFT was either done with dry technique [22, 23] or wet technique using tumescent solution with Ringer’s acetate, mepivacaine and adrenaline. AFT was performed under general anesthesia as outpatient procedures. Both groups underwent breast reconstruction with permanent silicone/saline expanders with detachable injection domes. The expanders were placed sub-pectoral with muscular coverage with access via the mastectomy scar and with a single pocket approach and the distal insertion of the muscle divided (intervention group at least 3 month after AFT) under general anesthesia. Different implants were used to be able to tailoring the reconstruction to the specific needs of the patients. One to seven doses of mepivacaine were given, 2 g pre and 2 g repeatedly post-operatively if the surgeon found it necessary. Specialized nurses performed all the postoperative expansions. After completed size adjustments the injection dome was removed. Following expander surgery all patients had follow-up appointments with the surgeon after 6, 12 and 24 months. The patients also saw the nurses for all minor problems or complications and surgeons were consulted if necessary. If the patients had a more serious complication or had to discuss further surgery, they saw the surgeons.
Primary outcomes: number of patients with and rate of complications and reoperations from first operation (intervention group: AFT; control group: expander insertion) up to 2 years after expander surgery. Complications included pneumothorax, laryngospasm, infection, contracture and seroma. Reoperations included all unscheduled operations after expander surgery, breast nipple reconstruction and removal of injection dome not included.
Secondary outcomes: total number of days of hospitalization due to AFT, expander surgery, reoperations and complications; number of visits to the outpatient clinic to surgeon and nurse, respectively. after the first operation (including the pre-operative consultations before a second AFT if needed and expander surgery for the intervention group). PRO was assessed with the first edition of the Breast-Q reconstruction module [18]. Eight domains of Breast-Q were analyzed Tables 5–7. They were compared between the groups at three time points and over time within each group. Baseline was compared to 6 and 24 months follow-up (referred to as baseline vs. 6, baseline vs. 24 and 6 vs. 24). Baseline was before any surgery (AFT or expander). Three domains considered quality of life and five considered satisfaction with reconstruction results and with care. The results are given as scores ranging from 0 to 100. A change of 5 to 10 on the scale is regarded as ‘a little’ change, 10 to 20 as ‘a moderate’ change and more than 20 as ‘very much’ change [24].
It was estimated that 80% of patients who underwent expander breast reconstruction had to undergo additional reoperations. To detect a decrease to 40% with 80% power at a significant level of 0.05, the minimum sample size was calculated to be 44 patients. We chose to add 10 patients to that number for our target sample size. At breast reconstruction consultations the plastic surgeons invited the patients eligible to the study. The enrollment was carried out by one of the two treating plastic surgeons. A research nurse carried out the randomization by blocking; allocations were equally divided into intervention and control in blocks of four. The statistician was blinded to allocation group. Allocation concealment to patients, surgeons and nurses was not possible. The characteristics of participants are described in Table 1. Continuous variables that are normally distributed are presented as mean with standard deviation. The continuous variables that are not normally distributed are shown as median with inter quartile range (IQR). Categorical variables are listed as number of cases with proportions in each group. Different indications and treatment types of complications and reoperations in the two intervention groups are shown in Table 2. The number of reoperations and complications were compared between the groups by Fisher’s exact test. Using two-sample Mann–Whitney test, the numbers of visits to a nurse and to a surgeon were compared. The number of days hospitalized during AFT and prosthesis surgery, and the number of days during the whole study period, were presented and compared between the two randomized groups, by using chi-square test. The mean score of the breast-Q in different domains were described, and the difference of the score between the two randomized groups were calculated (with 95% confidence interval [CI]). The Breast-Q scores were also analyzed longitudinally by comparing results at baseline with those at 6 and 24 months. The crossover comparisons were made in the two randomized groups. Intention-to-treat principle was applied in all the above-mentioned analyzes. The significant level was set as p-values less than 0.05. All the analyzes were conducted by using Stata MP 15.1 (StataCorp; College Station, Texas, USA).
| AFT group | Control group | |||
| Median/n | IQR | Median/n | IQR | |
| Age at reconstruction (years) | 58.6 | 14.4 | 56.7 | 16.3 |
| Radiation dose (Gy) | 50 | 0 | 50 | 0 |
| Anti-hormone therapy at baseline | 19 | 15 | ||
| Diabetes mellitus type II. Rheumatic decease | 2 | 2 | ||
| BMI at baseline | 25 | 4.9 | 24 | 3.8 |
| BMI after 2 years | 25.4 (n = 15) | 5.0 | 23.4 (n = 17) | 3.5 |
| Time from mastectomy to first reconstructive surgery (years) | 2.4 | 1.1 | 2.5 | 2.2 |
| Time from radiotherapy to first reconstructive surgery (years) | 1.9 | 1.7 | 2.1 | 2.3 |
| AFT | ||||
| Transplanted fat volume (cc) | 135.0 (64–275) | 70.8 | ||
| Patients undergoing two AFT sessions (n) | 5 | |||
| Type of implant | ||||
| Mentor Siltex Contour Profile Becker 35 | 14 | 13 | ||
| Allergan Natrelle 150 SH | 5 | 9 | ||
| Mentor Siltex contour 8100 Low height | 1 | 0 | ||
| Mentor CPG 323 | 1 | 0 | ||
| Volume of expander saline + gel (cc) | ||||
| At surgery | 225 | 100 | 223 | 69 |
| Maximum expansion | 368 | 133 | 358 | 134 |
| Final | 310 | 120 | 273 | 98 |
| Antibiotics dosage* (g) | 1 (1–5) | 3 | 1 (1–7) | 2 |
| Contralateral surgery during implant surgery | 13 | 15 | ||
| Contralateral surgery after implant surgery | 3 | 6 | ||
| Breast cancer recurrence | 4 | 1 | ||
| IQR: inter quartile range. | ||||
| *Per-operative at implant surgery and post-operative the following days if the surgeon found it necessary. | ||||
| Complications | AFT group | Control group | Reoperations | AFT group | Control group |
| Type | Indications | ||||
| Pneumothorax* | 1 | 0 | Asymmetry | 11 | 16 |
| Laryngospasm* dental injury | 0 | 1 | Contracture | 1 | 5 |
| Infection | 8 | 11 | Unevenness | 1 | 3 |
| Suspected infection | 1 | 3 | Infection | 1 | 1 |
| Contracture | 1 | 5 | Pain | 0 | 1 |
| Seroma | 3 | 2 | Other aesthetic reasons | 3 | 3 |
| All | 14 | 20 | Other reasons | 1** | 2*** |
| Treatment | Intervention | ||||
| Drainage | 2 | 1 | Implant replacement | 11 | 17 |
| Surgery | 2 | 4 | Capsulotomy | 10 | 14 |
| No intervention | 1 | 0 | AFT | 5 | 10 |
| Antibiotics. oral | 6 | 11 | Scar excision | 4 | 8 |
| Antibiotics. intravenous | 3 | 3 | Abdominal advancement | 1 | 6 |
| Implant extraction | 2 | 3 | |||
| Suture of sub-mammary fold | 4 | 2 | |||
| All | 37 | 60 | |||
| *During expander implant surgery. **Expander extraction for psychological reasons. ***Leakage from expander implant and problems with the fill tube. | |||||
Ethics
All patients gave informed consent. The Ethical Review Board in Stockholm approved the study. Ethical clearance number 2010/2072-31/3.
Results
The patients were included between 15 of December 2012 and 17 of September 2017. Due to decreased inclusion rate the inclusion was closed after 50 patients. Twenty-five patients were randomly assigned to each group. In the intervention group, 23 patients underwent AFT, 21 patients underwent expander surgery. In the control group, 22 patients underwent expander surgery (Figure 1). Table 1 shows baseline demographic and clinical characteristics.
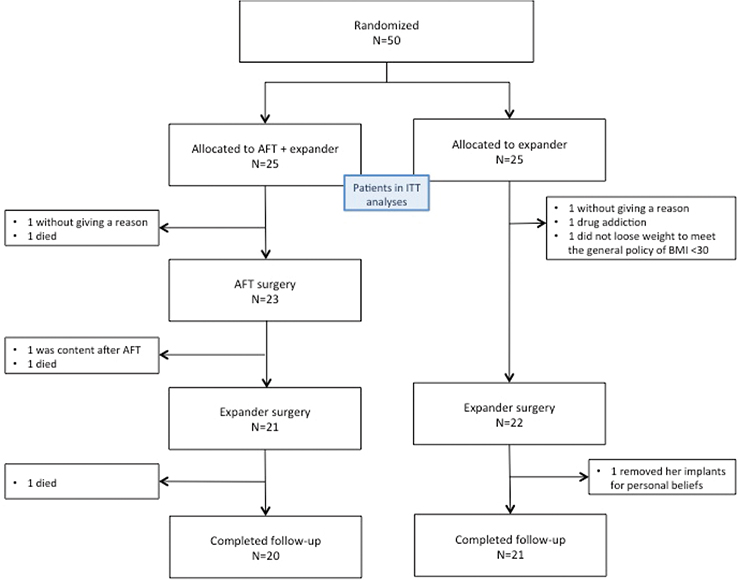
Figure 1. Flow diagram showing the recruitment of the participants in the study.
AFT: autologous fat transplantation.
Primary outcomes
Fifty-two per cent of the patients in the intervention group and 68% of the controls underwent at least one reoperation (p = 0.611). Twelve patients in the intervention group and nine of the controls underwent one reoperation. One and seven patients, respectively, underwent two reoperations and none in the intervention group and 1 of the controls underwent three reoperations (p = 0.132) (Table 3). The most common indication was asymmetry. The most common interventions were replacement of expanders followed by capsulotomy (Table 2). Thirty-two per cent of the patients in the intervention group and 52% of the controls had at least one complication (p = 0.347). Five patients in the intervention group and nine of the controls had one complication. Two and three patients, respectively, had two complications and one in each groups had three complications (p = 0.713) (Table 3). The most common complication was infection. Complications that led to reoperation were two in the intervention group and four in the control group (Table 3).
Secondary outcomes
The median number of medical consultations with the nurse was 4 (IQR 2) in the intervention group and six (IQR 3) in the control group (p = 0.002). Ten of the patients in the intervention group and three of the controls had 1–4 visits. Nine in the intervention group and 18 of the controls had 5–9 visits. One of the controls had >10 visits (p = 0.027) (Table 3). In median 42.5 mL of saline was installed or removed per visit in the intervention group and 25.7 in the control group. The median of number of medical consultations with a surgeon was 5 (IQR 3) in the intervention group and five (IQR 2) in the control group (p = 0.961). Four of the patients in the intervention group and six of the controls had 1–4 visits. Fifteen patients in each group had 5–9 visits. One of the controls had >10 visits (p = 0.617) (Table 3). About 75% of the patients spent 3–6 days in hospital during the surgery and 4–10 days totally. There were no statistically significant differences between the groups neither for the surgery alone, nor total days (Table 4).
Patient-related outcome – Quality of life: Psychosocial well-being: There was No difference in between the groups at baseline but at 24 months the difference was ‘moderate’, 16.93 (95% CI 4.10–29.75). Comparing baseline versus 6, both groups showed a ‘moderate’ change but comparing baseline versus 24, the intervention group showed significantly ‘very much’ change, 27.53 (95% CI 16.84–38.22) whereas the controls showed a non-significant ‘little’ change, 9.0 (95% CI –0.04 to 18.04). Sexual well-being: The analyzes showed no difference between the groups at baseline 0,6 (95% CI –8.9 to 10.1) but at 24 months there was a non-significant ‘moderate’ difference, 12.9 (95% CI –6.2 to 32.1). Comparing each group over time we found significant changes in both groups, both at 6 and 24 months, ‘moderate’ in the controls and ‘very much’ in the intervention group. The change for baseline versus 24 in the intervention group was 32.8 (95% CI 18.9 to 46.7) and for the controls 11.1 units (95% CI 3.9 to 18.4). Physical well-being chest: Change was shown neither between the groups nor over time within the groups (Table 5).
Patient-related outcome – Satisfaction: Satisfaction of outcome: There was a ‘little’ difference after 6 months, 5.1 (95% CI –9.7 to 19.9), after 24 months it was ‘moderate’, 13.2 (95% CI –2.8 to 29.1), although one of them significant. Comparing 6 versus 24 showed no difference in the intervention group but the controls showed a ‘little’ decline, -8.5 (95% CI –15.7 to 1.3). Satisfaction with the breast: There was No difference between the groups at baseline, –2.1 (95% CI –10.4 to 6.2). After 24 months the difference was ‘moderate’ 12.54 (95% CI 2.66 to 22.43). In baseline versus 6 AFT had a ‘moderate’ change and the controls had a ‘little’ change that persisted in baseline versus 24 months 9.25 (95% CI 2.63 to 15.87). The intervention group had a ‘very much’ change in baseline versus 24, 21.33 (95% CI 11.92 to 30.75). Satisfaction with breast nipples after 24 months showed a non-significant difference of 14.9 (95% CI –3.8 to 33.6) between the groups (Table 6). There were no differences in satisfaction with nipples, surgeon, medical team, and information between the groups.
Harms: One patient had a pneumothorax that was conservatively treated, and one had a laryngospasm that led to a dental injury during the expander surgery. None of these adverse events was related to the intervention of the study.
Discussion
When conducting studies of this kind, power calculations are challenging and easily err. We aimed to include 10 patients more than we needed according to the calculation. Inclusion rate decreased over time, and we had more drop-outs than expected. The main reason of the decrease were increased availability of increased use of primary breast reconstruction. Nevertheless, the analyzes point in the same direction despite few patients. Not being able to blind the surgeon and patient is a limitation. Both the patients and the surgeons are aware of the aim of the study, and this can bias especially the aesthetic indications for reoperations and results of PRO. A longer follow-up period for measuring PRO might provide clearer results, but concerning the primary outcomes it has previously been shown that 2 years follow-up are enough [25]. At the time of the study, Breast-Q was not validated for Swedish women. A limitation of this study is the study size. The generalizability for the primary outcomes is high due to randomization. Even if the complication and reoperation panorama in this cohort would differ from other cohorts, the differences shown between the two groups are most likely applicable in other settings. The inclusion criteria were wide meaning that the favorable study results may be applicable to many patients who undergo mastectomy and radiotherapy and where expander breast reconstruction then can be considered as an option. The results of PRO and numbers of appointments at the outpatient clinic should vary equally in both groups and not affect the generalizability. Primary outcomes were reoperations and complications. Since there were no comparable studies the power calculation had to be hypothetic and hence underestimated the number of patients needed to detect the effect size of AFT that can be seen in this study. The results are in favor of the AFT in the primary outcomes. Fewer patients who underwent AFT prior to breast reconstruction with expanders needed any reoperation. The patients who needed any reoperation underwent fewer operations. The AFT patients generally needed one operation whereas almost half of the controls underwent two operations. More than half of the patients in the AFT group did not have any complication, but more than half of the controls did have one or more complications, infection being the most common and treated with oral antibiotics. There was a tendency that AFT had a positive effect on the postoperative succession of events, even if it was not significant in other aspects than the number of appointments. The postoperative appointments were mostly to specialist nurses and the patients only met the surgeon at the scheduled follow-ups or if a nurse had medical doubts and therefore consulted the surgeon. Eighty-six per cent of the controls needed five or more appointments with nurse compared to 43% of the AFT group (p = 0.002), which demonstrate the need of fewer expansions with larger saline volumes and less general troubles. Comparing how many days the patients were hospitalized further supports the positive tendency in favor of AFT. Most of the AFT patients (33%) stayed 2 days in hospital after their expander surgery while most of the control patients (36%) stayed for 3 days. The analysis of total days in hospital showed no statistical difference but 32% of the patients in the control group stayed 10 or more days compared to 9.5% in the intervention group (Table 4). To summarize, there is a tendency that our hypothesis that AFT to the irradiated tissue prior to expander surgery reverses some of the negative effects of radiotherapy. All patients were satisfied with their reconstructed breasts and both the psychosocial and sexual well-being were improved at the follow-up. In all items the AFT patients had a better progression overtime than the controls. When comparing the two groups after 24 months the AFT patients were significantly more satisfied than the controls with both the reconstructed breasts and the psychosocial well-being. The same result was seen for outcome and sexual well-being, but we had to few observations for significance. Overall, the AFT patients scored higher, post- but not pre-operatively than the control patients (Figure 2). On the contrary the two groups scored uniformly in the items of satisfaction with information, surgeon, medical team, and office staff, which indicates that there is little bias associated with the intervention (Table 7). The intervention group was not more satisfied even though they were randomized to extra treatment. In conclusion we found that AFT decrease postoperative visits and increased satisfaction and well-being. We consider that AFT prior to breast reconstruction with expander may be a possible choice for irradiated women who are not suitable for or willing to undergo extensive surgery. With AFT as a part of the procedure, even more women may benefit from breast reconstruction, enhancing their psychosocial well-being.
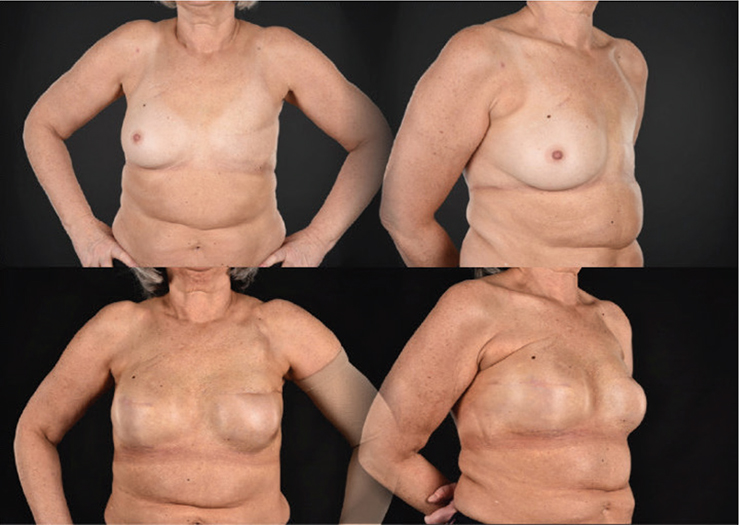
Figure 2. (A and B). Preoperative photo. A 61-year-old woman previously treated with mastectomy and radiotherapy due to breast cancer. Two-year postoperative photo. Autologous fat transplantation with 180 mL fat prior to expander surgery. She had one reoperation with change of implant and a capsulotomy during follow-up. Contralateral: risk-reducing mastectomy with immediate reconstruction.
Acknowledgments
The study is registered at ClinicalTrials.gov PRS, ID number NCT02637635. This work was supported by the Stockholm County Council (ALF project) under Grant number 20170121; Capio Research Foundation and Percy Falk's Foundation (Percy Falks stiftelse för forskning beträffande prostatacancer och bröstcancer) under Grant number K112120033.
ORCIDs
Anna Lindegren  https://orcid.org/0000-0003-0800-4375
https://orcid.org/0000-0003-0800-4375
Inkeri Schultz  https://orcid.org/0000-0002-3218-0881
https://orcid.org/0000-0002-3218-0881
Åsa Edsander-Nord  https://orcid.org/0000-0002-3218-0881
https://orcid.org/0000-0002-3218-0881
Jacinth Yan  https://orcid.org/0000-0002-3218-0881
https://orcid.org/0000-0002-3218-0881
Marie Wickman  https://orcid.org/0000-0002-3218-0881
https://orcid.org/0000-0002-3218-0881
References
[1] Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011; 378(9804): 1707–1716. https://doi.org/10.1016/S0140-6736(11)61629-2
[2] McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014; 383(9935): 2127–2135. https://doi.org/10.1016/S0140-6736(14)60488-8
[3] Taylor C, Correa C, Duane FK, et al. Estimating the risks of breast cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017; 35(15): 1641–1649. https://doi.org/10.1200/JCO.2016.72.0722
[4] Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park, NY). 2017; 31(12): 885–887, 94–99.
[5] Sjövall K, Strömbeck G, Löfgren A, et al. Adjuvant radiotherapy of women with breast cancer – information, support and side-effects. Eur J Oncol Nurs. 2010; 14(2): 147–153. https://doi.org/10.1016/j.ejon.2009.09.002
[6] Straub JM, New J, Hamilton CD, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015; 141(11): 1985–1994. https://doi.org/10.1007/s00432-015-1974-6
[7] Eriksson M, Anveden L, Celebioglu F, et al. Radiotherapy in implant-based immediate breast reconstruction: risk factors, surgical outcomes, and patient-reported outcome measures in a large Swedish multicenter cohort. Breast Cancer Res Treat. 2013; 142(3): 591–601. https://doi.org/10.1007/s10549-013-2770-0
[8] Lee BT, Adesiyun AT, Colakoglu S, et al. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plastic Surg. 2010; 64(5): 679–683. https://doi.org/10.1097/SAP.0b013e3181db7585
[9] Liljegren G, Holmberg L, Westman G. The cosmetic outcome in early breast cancer treated with sector resection with or without radiotherapy. Uppsala-Orebro Breast Cancer Study Group. Eur J Cancer (Oxford, England: 1990). 1993; 29a(15): 2083–2089. https://doi.org/10.1016/0959-8049(93)90038-H
[10] Benediktsson K, Perbeck L. Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plastic Reconstruct Aesthetic Surg. 2006; 59(1): 27–34. https://doi.org/10.1016/j.bjps.2005.08.005
[11] Zugasti A, Hontanilla B. The impact of adjuvant radiotherapy on immediate implant-based breast reconstruction surgical and satisfaction outcomes: a systematic review and meta-analysis. Plastic Reconstruct Surg Glob Open. 2021; 9(11): e3910. https://doi.org/10.1097/GOX.0000000000003910
[12] Coudé Adam H, Frisell A, Liu Y, et al. Effect of radiotherapy on expanders and permanent implants in immediate breast reconstruction: long-term surgical and patient-reported outcomes in a large multicentre cohort. Br J Surg. 2021; 108(12): 1474–1482. https://doi.org/10.1093/bjs/znab333
[13] Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg. 2009; 33(5): 695–700. https://doi.org/10.1007/s00266-009-9366-4
[14] Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic Reconstruct Surg. 2007; 119(5): 1409–1422; discussion 23–24. https://doi.org/10.1097/01.prs.0000256047.47909.71
[15] Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plastic Reconstruct Surg. 2012; 129(2): 317–29. https://doi.org/10.1097/PRS.0b013e31822b6619
[16] Lindegren A, Schultz I, Sinha I, et al. Autologous fat transplantation alters gene expression patterns related to inflammation and hypoxia in the irradiated human breast. Br J Surg. 2019; 106(5): 563–573. https://doi.org/10.1002/bjs.11072
[17] Salgarello M, Visconti G, Farallo E. Autologous fat graft in radiated tissue prior to alloplastic reconstruction of the breast: report of two cases. Aesthetic Plast Surg. 2010; 34(1): 5–10. https://doi.org/10.1007/s00266-009-9367-3
[18] Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plastic Reconstruct Surg. 2009; 124(2): 345–353. https://doi.org/10.1097/PRS.0b013e3181aee807
[19] Razzouk K, Fitoussi A, Al Khori N, et al. Breast reconstruction combining lipofilling and prepectoral prosthesis after radiotherapy. Plastic Reconstruct Surg Glob Open. 2020; 8(5): e2659. https://doi.org/10.1097/GOX.0000000000002659
[20] Sarfati I, Ihrai T, Kaufman G, et al. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J Plastic Reconstruct Aesthetic Surg. 2011; 64(9): 1161–1166. https://doi.org/10.1016/j.bjps.2011.03.031
[21] Lesniak DM, Sarfati I, Meredith I, et al. Fat Grafting before delayed prophylactic mastectomy and immediate implant reconstruction for patients at high risk of complications. Plastic Reconstruct Surg. 2022; 149(1): 52–56. https://doi.org/10.1097/PRS.0000000000008672
[22] Lindegren A, Chantereau MW, Bygdeson M, et al. Autologous fat transplantation to the reconstructed breast does not hinder assessment of mammography and ultrasound: a Cohort study. World J Surg. 2016; 40(5): 1104–1111. https://doi.org/10.1007/s00268-015-3385-x
[23] Lindegren A, Schultz I, Wickman M. Improved patient-reported outcomes after autologous fat transplantation and corrective surgery after breast surgery. J Plastic Surg Hand Surg. 2019; 53(2): 111–118. https://doi.org/10.1080/2000656X.2018.1561456
[24] BREAST-Q Q-Score manual. Users’ manual version 1.0 July 2012. Memorial Sloan Kettering Cancer Centerand The. University of British Columbia © 2006, All rights reserved, New York.
[25] Roberts A, Baxter N, Camacho X, et al. Once is rarely enough: a population-based study of reoperations after postmastectomy breast reconstruction. Ann Surg Oncol. 2015; 22(10): 3302–3307. https://doi.org/10.1245/s10434-015-4716-8