ORIGINAL RESEARCH ARTICLE
Systematic review of cost-effectiveness in breast reconstruction: deep inferior epigastric perforator flap vs. implant-based breast reconstruction
Emma Hanssona,b, Fredrik Brorsona,b, Jonas Löfstranda,b, Anna Elandera,b and Mikael Svenssonc,d
aDepartment of Plastic Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bDepartment of Plastic and Reconstructive Surgery, Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; cDepartment of Pharmaceutical Outcomes & Policy, College of Pharmacy, University of Florida, Gainesville, FL, USA; dSchool of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
ABSTRACT
Background: There are several techniques for reconstructing breasts after mastectomy, but little scientific evidence for which technique is superior. The aim of this systematic review was to compare the cost-effectiveness of implant-based and autologous reconstruction and to evaluate the overall certainty of evidence, as well as the quality of reporting of the included studies.
Methods: Studies investigating the cost-effectiveness of breast reconstruction with a deep inferior epigastric perforator (DIEP) flap compared to implant-based reconstruction, meeting criteria defined in a PICO (population, intervention, comparison, and outcome), were included. Medline, PubMed, Embase, Cochrane library, CinahL, EconLit, and NHS EED databases were searched. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of evidence, and the Consolidated Health Economic Evaluation Reporting Standard (CHEERS) 2022 was used to evaluate the quality of reporting.
Results and conclusions: A total of 256 abstracts were retrieved from the search, and after scrutiny, seven studies were included. The findings of this present systematic review should be interpreted with caution as the overall certainty of evidence is low (GRADE ƟƟОО). The included studies suggest that DIEP-flaps are cost-effective compared with implant-based breast reconstruction when the applied cost-effectiveness thresholds of $50,000 to $100,000 per quality-adjusted life years are used. It is noteworthy that no high level evidence exists regarding cost-effeciency, to support recommendations and decision in breast reconstruction. Methodological issues that can be improved in future studies are presented.
KEYWORDS: Health economics; breast reconstruction; plastic surgery; ICER; cost-effectiveness; prioritizing
Citation: Journal of Plastic Surgery and Hand Surgery 2024; 59: 159–171. DOI: https://doi.org/10.2340/jphs.v59.19649.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 24 September 2023; Accepted: 21 November 2023; Published: 8 January 2024.
CONTACT Emma Hansson emma.hansson.2@gu.se Department of Plastic Surgery, Sahlgrenska University Hospital, Gröna Stråket 8, SE-413 45 Gothenburg, Sweden. Tel: +46313421000/Fax: 031 82 79 03
Supplemental data for this article can be accessed online at https://doi.org/10.2340/jphs.v59.19649
Competing interests and funding: The authors declare that they have no conflict of interest.
Introduction
There are several techniques for reconstructing breasts after mastectomy, but little scientific evidence for which technique is superior, and guidelines and praxises are varying [1]. Core outcomes for breast reconstruction include complications, reoperations, donor site morbidity, quality of life, and patient satisfaction [2]. However, in healthcare systems with limited resources, these variables have to be combined with costs, when different methods are compared [3]. Most previous health economic evaluations of different methods for breast reconstruction have used cost-minimization analysis, that is they have presented costs without taking benefits for the patients into consideration [4–10]. Such a methodology assumes that the patient benefit is identical for the different treatment options that are compared, which does not seem to be a valid assumption for breast reconstruction techniques [11–13].
In a cost-effectiveness analysis (CEA), both costs and patient benefits are combined [14]. The difference in costs between two methods is called the incremental cost, whereas the difference in patient benefits is called the incremental effectiveness; the ratio between them is the incremental cost-effectiveness ratio (ICER). Effectiveness with regard to quality of life outcomes is often expressed in generic terms, in the form of quality-adjusted life years (QALYs), which is the combination of life-years and the QALY-weight in each time period (also called utility-scores or health-related quality of life (HRQoL) scores). The latter is generated from generic quality of life instruments, as apposed to disease specific instruments, or directly from preference values assessed using time trade-off or standard gamble approaches. Examples of generic instruments include the Medical Outcomes Study 36-Item Short Form (SF-36) Health survey/RAND 36-Item Short Form Health Survey [15,16], Health Utilities Index (HUI) [17], and the EuroQol Instrument (EQ-5D) [18]. QALY-weights (utility scores) provide a method to compare different health states on a common interval scale of 0–1, where 0 indicates death and 1 indicates perfect health, that is a more preferred health state will receive a greater weight [19,20].
There is a myriad of different surgical options to reconstruct breasts, such as different meshes and implants, as well as different pedicled or free flaps. However, the two main categories are implant-based and autologous techniquess. The most common autologous technique is the deep inferior epigastric perforator (DIEP) flap. Few studies [4,21] combine costs and effectiveness to compare the two methods directly, and the overall certainty of evidence has never been evaluated.
The aim of this systematic review was to compare the cost-effectiveness of implant-based vs. autologous reconstruction and to evaluate the overall certainty of evidence, assessed according to the GRADE approach [22], as well as the quality of reporting of the included studies.
Methods
Protocol
This is a systematic review pre-registered in PROSPERO (CRD42023424375).
Eligibility criteria and study selection
Studies investigating the cost-effectiveness of breast reconstruction with a DIEP flap compared to an implant-based reconstruction were included. The articles had to meet criteria defined in a PICO (population, intervention, comparison, and outcome) [23]. P: Women who seek health care for breast reconstruction following mastectomy, I: Breast reconstruction with a DIEP flap, C: Breast reconstruction with an implant-based technique, O: Incremental cost-effectiveness ratio. Eligible study designs were health economic evaluations fulfilling the PICO. Editorials, letters, and systematic reviews were excluded. The authors independently assessed whether the articles met the inclusion criteria, and disagreements were resolved by discussion.
Information sources and search strategy
Medline, PubMed, Embase, Cochrane library, CinahL, EconLit, and NHS EED databases were searched on 09.05.2023 for articles and abstracts, without time limit. No grey literature sources were searched. The search was limited to studies published in English, French, Italian, Swedish, Danish, and Norwegian. The search string were ((((cost effective) OR (cost utility)) OR (economic evaluation)) AND (breast reconstruction)) AND (((DIEP) OR (deep inferior epigastric perforator flap)) OR (autologous)). The full-text article was read when eligibility for inclusion could not be assessed by reading the abstract. Disagreements were resolved by discussion.
Data abstraction
Information collected included first author, year of publication, study country, interventions and comparators, sample size, demographic data of included patients and controls, perspective, costs, type of study, modeling approach, time horizon, year of costing, ICER, sensitivity analyses, and study conclusions.
Quality assessment
The GRADE approach was used to assess the certainty of evidence [24,25]. Within and across studies, risk of bias (study limitations), inconsistency of results (unexplained heterogeneity), indirectness of evidence, and imprecision were evaluated as ‘low’, ‘unclear’, or ‘high’ [26]. The overall certainty of evidence was rated down based on the assessment of risk of bias, indirectness, inconsistency, and imprecision according to the GRADE manual and finally rated as ‘High’ (ƟƟƟƟ), ‘Moderate’ (ƟƟƟО), ‘Low’ (ƟƟОО), or ‘Very low’ (ƟООО) [22,26]. The Consolidated Health Economic Evaluation Reporting Standard (CHEERS) 2022 was used to evaluate the quality of reporting of the included studies [27].
Results
Overview of included studies
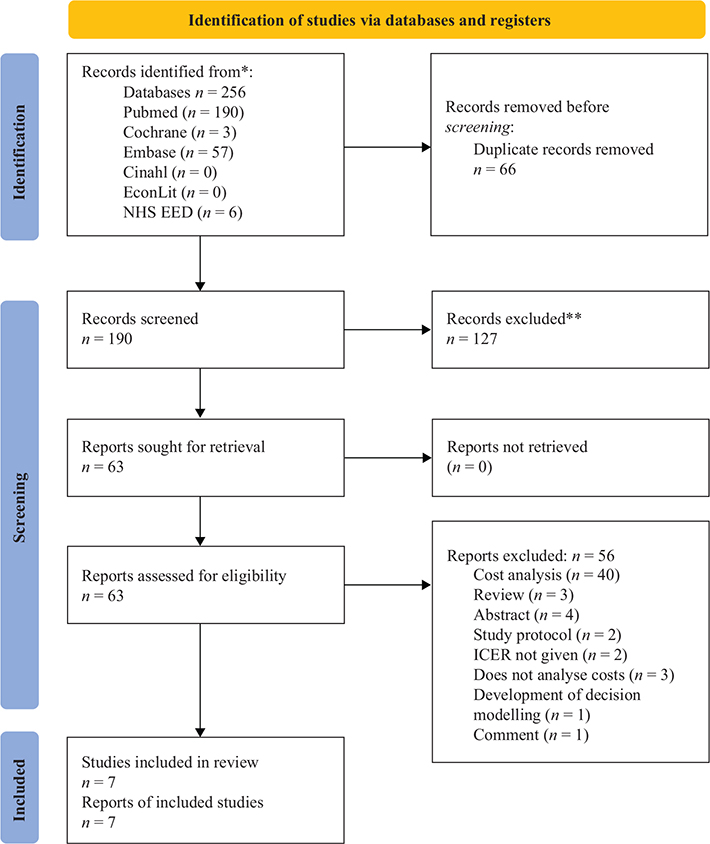
A total of 256 abstracts were retrieved from the search. Of these, 66 were removed for duplication, 127 were excluded as they did not meet the inclusion criteria, and 63 abstracts were included for next level of screening (Figure 1). After scrutiny of the full texts, 56 abstracts (Table S1) were excluded further, and finally seven studies were included in this review (Table 1).
| Author, year, country | Intervention vs. comparator | Patients | Mean age, years (SD) | Sample size | Perspective | Costs | Type of study | Method for sensitivity analysis | Time horizon | Year of costing | ICER (incremental cost/QALY*) (cost-effectiveness level – CE-level) | Threshold for cost-effectiveness | Result of Sensitivity analysis | Study conclusions |
| Bloom, 2023, USA [28] | Delayed-immediate DIEP vs. delayed-immediate implant | Locally advanced breast cancer (T3 N1-3) that require post- mastectomy radiation |
Hypothetical cohort of patients Base case 45 years old, unilateral BR, Life expectancy from the time of surgery 36.1 years |
NA | Health care (hospital costs) |
Based on Medicare CPT codes and DRG |
Modeling QALY calculated from utilities derived from previously published studies. Utilities without complications: DIEP – 0.83, Implant – 0.69 Probability of complications and corrections obtained from a nonsystematic literature review (no RCT). Varying study design and follow-up. |
Decision tree Sensitivity analysis: deterministic and probabilistic (Monte Carlo simulation) |
36 years | 2021 | Implant used as comparator DIEP $2141 CE-level: |
ICUR of less than $50,000 | Deterministic DIEP is the most cost-effective alternative if the cost of it is less than $257444.13 Probabilistic Monte Carlo simulation shows a confidence of 99.99% in favor of DIEP |
‘Despite the decreased donor-site morbidity, delayed immediate tissue expander/implant reconstruction has much a higher rate of complications, such as capsular contracture in irradiated fields, leading to decreased clinical effectiveness compared with DIEP reconstruction and pointing to the ultimate conclusion that using autologous tissue in irradiated fields is more cost-effective’ |
| Grover, 2013, USA [30] | Mastectomy only was compared to ‘autologous free flap’, expander-implant, and immediate implant | NR | Hypothetical cohort of patients Base case not reported. |
NA | Health care (hospital costs) |
Based on Medicare CPT codes and DRG |
Modeling QALYs calculated from utilitites derived from visual analogue scale (VAS) questionnaires to 9 plastic surgeons. Probability of complications and corrections obtained from a nonsystematic literature review. It is stated that the review was systematic, but methods for this and quality evaluation of the included articles are NR. Varying study design and follow-up. All levels of evidence were included from RCTs to case series. |
Decision tree Sensitivity analysis: Probabilistic. |
7 years | 2011 | Mastectomy only as comparator Free flap: $66,843 Immediate implant: $161,858 Expander-implant: $526,673 |
$100,000 per QALY | ±15% variation in costs, probabilities and utilities only changed preferences for pedicled without implants and free flaps, but not otherwise. Without radiotherapy costs changed the results. Changes in preferences changed the results. Probabilistic Monte Carlo simulation confirmed the results. |
The results ‘clearly favoured autologous reconstruction in both non-irradiated and irradiated patients’. |
| Klifto, 2021, USA [35] | Immediate DIEP vs. immediate direct to implant (DTI) and tissue expander to implant (TEI) Also included in analysis: Latissimus dorsi and implant, LD, Pedicled TRAM, Free TRAM, Thigh-based flap, Gluteal-based flap |
Localized breast cancer (stage 1 or 2) not requiring radiotherapy | Hypothetical cohort of patients Base case 45 years old, BMI 18.5–35, immediate breast reconstruction |
NA | Health care (hospital costs) and societal (production and petrol) |
Based on Medicare reimbursements, commercially a vailable industry data. Time off work was calculated using U.S. Census Bureau 2019 annual reports from full-time women ages 45–54 years. Petrol costs from 2020 US averages. |
Modeling QALYs calculated from utilities and PROMs derived from previously published studies. Optimal utilities used: Unilateral reconstruction without complications with DIEP – 0.854, DTI – 0.767, and TEI – 0.732. Bilateral with DIEPs – 0.874, DTIs – 0.787, and TEIs – 0.752 Probability of complications and corrections obtained from a nonsystematic literature review. Varying study design and follow-up. |
Markov modeling Future costs and utilities discounted at 3% Sensitivity analysis: Probabilistic |
10 years | 2021 |
LD provided the lowest overall cost and therefore used as a comparator. Hospital perspective Unilateral DIEP: $8307.65 DTI: -$42109.35 TEI: -$22036.02 Bilateral DIEPs $10956.63 DTIs -$41146.86 TEIs: $327161.41 Societal perspective Unilateral DIEP $7696.70 DTI: -$45760.94 TEI: -$23143.76 Bilateral DIEPs $10373.85 DTIs: -$44281.68 TEIs: -$28519.90 CE-level |
$50,000 per QALY | Unilateral cost-effectiveness for DIEP 32.7–34.4%, DTI 0.03–0.06%, TEI 0.1–0.12% Bilateral for DIEPs 35.9–36.8%, DTI 0.04–0.05%, TEI 0.02% |
‘LD provides the lowest overall costs, while DIEP/SIEA provides the greatest overall effectiveness and overall NMB [net monetary benefits]’. |
| Kouwenberg, 2021, the Netherlands [33] | Autologous free frlap vs. implant-based reconstruction Mastectomy only and breast conserving surgery also included in analysis. |
All patients operated on for breast cancer in the department, between January 1st, 2005 and January 1st, 2017 |
Age autologous: 48.2 years (SD 10.6), Implant: 49.2 (SD 10.6) |
Complications from 621 implant-based 513 autologous |
Health care | Dutch unit costs | QALY weights (EQ-5D-5L) generated from a previous study performed in the department. Nu differences between QALYs for different methods. Complications from included cohort. Only reoperations performed during the first 60 days? |
? |
Up to 10 years Follow-up of included cohort implant: 96.4 months (SD 48.8). Autologous: 86.9 (SD 50.8) |
2018 Costs discounted 4% and effects 1.5% per year |
Mastectomy only comparator Autologous EUR 51,715 Implant-based EUR 28,406 |
NR | Implant based reconstruction would still be more cost-effective if all implants had to be ex–changed in a 10 year perspective. If complications autologous free flaps were reduced by 40%, it would be below EUR 80,000/QALY and by 60% below EUR 50,000/QALY. |
Implant-based reconstruction seems to be more cost-effective due to a high complication-rate of autologous reconstruction |
| Matros, 2015, USA [32] | DIEP vs. implant | Unilateral and bilateral DIEP/s and implant/s | NR | PROMs from 309 patients operated at Memorial Sloan Kettering Cancer Center, New York NA for complications |
Health care (hospital costs) |
Obtained from the Nationwide Inpatient Sample Database (NIS) and DRG | Modeling Breast-QALYs were generated using a mean value from the six BREAST-Q domains, measured 1–8 years after the reconstruction in patients operated at Memorial Sloan Kettering Cancer Center (New York, NY). Breast health-related quality-adjusted lifeyears = Duration of health state Å~ Effectiveness of health state + (Number of healthy years remaining − Duration of health state) Å~ Effectiveness of successful breast reconstruction. Probability of complications and corrections obtained from a nonsystematic literature review. |
Decision tree Charges for complications in years 2 to 36 were discounted by patient life expectancy (3%) Sensitivity analysis: deterministic |
36 years | 2010 | Implants used as comparator Unilateral DIEP $11,941 Bilateral DIEPs $28,017 *Breast-QALYs CE-level: |
$50,000 per QALY | ‘Patients at earlier stages have a lower incremental cost-effectiveness ratio than patients with advanced breast cancer. For example, the cost of an additional breast health-related quality-adjusted life-year in a patient with stage 0 cancer who chooses unilateral mastectomy with DIEP flap reconstruction is $11,941. If the same patient is diagnosed with stage 4 cancer, the cost for an additional breast health-related quality-adjusted life-year is $142,667. A similar relationship was present for bilateral procedures’. |
‘The current data support the hypothesis that autologous reconstruction is worthwhile, especially in those with longer life expectancy, when both costs and quality of life are factored together’. |
| Razdan, 2016, USA [29] | Delayed DIEP vs. immediate implant based Also included in analysis: Mastectomy only |
Locally advanced breast cancer (stage 2 or 3) that require post- mastectomy radiotherapy, with an average life-expectancy of 7 years. | Hypothetical cohort of patients Base case 48 years old, life expectancy from the time of surgery 7 years |
PROMs from 343 patients (76 DIEPs, 196 implants, 71 mastectomies alone) operated at Memorial Sloan Kettering Cancer Center, New York NA for costs and complications revisions |
Health care (hospital costs) | Obtained from the Nationwide Inpatient Sample Database (NIS) | Modeling Breast-QALYs were generated using a mean value from the six BREAST-Q domains. NR at what time point it was measured. Probability of complications and corrections obtained from a nonsystematic literature review. Varying study design and follow-up. |
Decision tree model Sensitivity analysis: deterministic |
7 years | 2010 | Mastectomy alone used as comparator DIEP: $102,509 /QALY Implant: $57,906/QALY *Breast-QALT CE-level: |
$100,000 per QALY | ‘If greater life expectancy is anticipated, autologous transfer is cost-effective as well and may be a superior option because of greater associated long-term HRQOL’ | |
| Thoma, 2020, Canada [17] | Abdominally based free flap vs. expander to implant (ETI) | Patients requiring uni- or bilateral immediate or delayed reconstruction after mastectomy. | Age: 50 years (SD 8.9) BMI: 26.6 (SD 6) 14 unilateral and 31 bilateral reconstructions |
44 (19 abdominally based free flaps and 16 ETI | Health care and society | Hospital system. Productively loss and activities of daily living (ADL) due to the surgery were documented in diaries and assigned a monetary value according the Human Capital method. | Pragmatic prospective cohort study. QALY calculated from The Health Utilities Index Mark 3 (HUI-3) at baseline, and at 1, 6, and 12 months post-operatively. |
Bootstrapping | 1 year | 2016–2017 | ICER not calculated as free flaps are both less effective and more costly than ETI | $50,000 per QALY | Bootstrapping? | In the first post-operative year, a free flap is not cost-effective when compared to expander to implant when a generic quality of life instrument is used. |
| BMI: body mass index; BR: breast reconstruction; CPT: current procedure terminology; DIEP: deep inferior epigastric perforator flap; DRG: diagnostic-related group; DTI: direct to implant; HRQOL: health-related quality of life; LD: latissimus dorsi flap; N: node stage; NMB: net monetary benefit; NA: not applicable; NIS: Nationwide Inpatient Sample Database; NR: Not reported; PROM: patient reported outcome measurement; QALY: quality-adjusted life years; RCT: randomized controlled trial; TEI: tissue expander to implant; T: tumor stage; TRAM: transverse rectus abdominis myocutaneous flap. | ||||||||||||||
All of the included studies compared DIEP with implant-based reconstruction, but the study objectives varied slightly. Two studies only included radiated patients [28,29] and one compared radiated and nonradiated patients [30], whereas the other studies did not report the variable radiation. Most studies did not define the tumor stage of the patients, while two studies only included patients with locally advanced cancer (T3 N1-3 M0 [28] and T2-3 [29]) and one localized breast cancer [31], which gave different life expectancies. The other studies did not report types of cancer or subgroups. Similarly, most studies did not define if the reconstruction was performed as an immediate or a delayed procedure, while one study compared delayed DIEP with immediate implant-based reconstruction [29].
The time horizon of the studies varied; three studies based it on a lifetime perspective, which is the life expectancy of the included patients [29,32], whereas three studies did not define why the time horizons, 10 [31,33] and 7 [30] years, were chosen. One study had a 12-month perspective, as it was realistic for patient follow-up [34].
Five studies were performed in the US healthcare system, one in the Dutch system [33], and one in the Canadian system [17]. Hence, most of the studies were performed in systems where a universal coverage is not provided (n=5). Five studies had a healthcare perspective, including hospital costs, whereas two studies also adopted a societal perspective and included costs of loss of production [17,31].
Five studies were based on modeling, of which four used decision trees [28–30,32], and one Markov modeling [31], and two were performed alongside nonrandomized clinical cohort studies: one prospective [17] and one retrospective [33]. In the studies based on modeling, the complications were derived from previously published studies, based on nonsystematic literature reviews. QALYs were calculated from QALY-weights/utility-scores derived from previously published preferences, and extracted preferences in two studies [28,35], a breast-specific quality of life questionnaire (BREAST-Q, n = 309 + 343) in two studies [29,32], generic quality of life questionnaires in two studies (EuroQol 5 dimensions (EQ5D), n = 1871 and Health Utilities Index Mark 3 (HUI-3), n = 44) [17,36], and preferences elicited directly from nine plastic surgeons in one study [30].
Main findings of the included studies
Five studies suggested that, based on a cost-effectiveness threshold of $50,000 to $100,000 per QALY, breast reconstruction with a DIEP might be cost-effective as compared with implant-based breast reconstruction. However, two of the studies commented that implant-based reconstruction might be more effective clinically in cases where a short life-expectancy is anticipated [28,29], while one study reported that DIEP is particularly cost-effective in patients where a long life-expectancy is anticipated [32]. Two studies concluded that implant-based reconstruction is more cost-effective, one due to a high complication rate in autologous reconstruction [33], and one with a 1-year perspective [17]. Both studies used generic quality of life instruments to measure benefits.
Quality assessment of the included studies
Risk of bias
The credibility of a model is dependent on the certainty of evidence for each model input [25] (Table 2). Effectiveness was measured in the form of QALYs in all studies, but the way QALY-weights were elicited varied considerably. Only one of the studies elicited QALY-weights and the complications and costs from the same patients [17]. Two studies [28,31] used previously published QALY-weights. However, the preference values existing for health states requiring breast reconstruction and breast reconstruction are of poor scientific quality [37]. In one study [30], utilities based on visual analogue scale questionnaires to nine plastic surgeons were used. Hence, the patients’ opinions were not included. In the other four studies, QALY-weights were based on patients’ answers to health-related quality of life instruments. Two studies used a breast-specific instrument and two used generic instruments. There is no validated way to calculate QALY-weights from the breast specific instrument (‘breast-QALYs’), and it has been suggested that generic instruments are too general to capture differences between methods to reconstruct breasts [11,38]. In addition, in all but one [33] of the studies, the characteristics of the populations on which the QALY-weights were based were not described. The studies that have used previously published QALY-weights or preferences to calculate QALYs have not described how the data were selected, extracted, and synthesized. The studies that elicited QALY-weights from patients did not describe how the patients were included, if there were any exclusions, at what time point the measurements were performed, and the demographics of the groups. Hence, the representativeness and validity of the samples and the way QALYs were calculated in the models cannot be properly evaluated.
| GRADE [24,25] | Study | ||||||
| Bloom, 2023, USA [28] | Grover, 2013 [30] | Klifto, 2021, USA [35] | Kouwenberg, 2021 [33] | Matros, 2015, USA [32] | Razdan, 2016, USA [29] | Thoma, 2020 [17] | |
| Risk of bias | |||||||
| QALYs | - Low-quality of previously published preferences [37] Characteristics of previously published populations on which QALYs are based are not described. |
- Elicited from nine plastic surgeons. Patient perspective not included. |
- Extracted from previous studies, without systematic review and without quality evaluation. Not stated how summary QALY was calculated. Characteristics of previously published populations on which QALYs are based are not described. |
? Differences between breast reconstruction methods cannot be detected with generic quality of life instruments [11]? Not collected alongside RCT. |
? No validated method to calculate ‘breast-QALYs’ Characteristics of previously published populations on which QALYs are based are not described |
? No validated method to calculate ‘breast-QALYs’ Characteristics of previously published populations on which QALYs are based are not described |
- Differences between breast reconstruction methods cannot be detected with generic quality of life instruments [11]? Not collected alongside RCT Only 35 patients in cohort Only BMI, age, and laterality are described for the cohort. |
| Probability of complications | - Not based on systematic review and no inclusion and exclusion and search criteria. No quality evaluation of included studies. Not reported how previous studies have been synthesized. Characteristics of populations on which complications are based are not described. |
- Not based on systematic review and no inclusion and exclusion and search criteria. No quality evaluation of included studies. Not reported how previous studies have been synthesized. Characteristics of populations on which complications are based are not described. |
- Not based on systematic review and no inclusion and exclusion and search criteria. No quality evaluation of included studies. Not reported how previous studies have been synthesized. Characteristics of populations on which complications are based are not described. |
? Not reported how data on complications were collected and how they were defined. Only re-operations performed during the first 60 days included. |
- Not based on systematic review and no inclusion and exclusion and search criteria. No quality evaluation of included studies. Not reported how previous studies have been synthesized. Characteristics of populations on which complications are based are not described. |
- Not based on systematic review and no inclusion and exclusion and search criteria. No quality evaluation of included studies. Not reported how previous studies have been synthesized. Characteristics of populations on which complications are based are not described. |
? Only 35 patients in cohort. Not reported how data on complications were collected and how they were defined. |
| Costs | + | + | + | + | + | + | + |
| Influence of different variables – sensitivity analysis | ? Uncertain measurements of variabilities in complications and QALYs |
? Uncertain measurements of variabilities in complications and QALYs |
? Uncertain measurements of variabilities in complications and QALYs |
? Uncertain measurements of variabilities in complications and QALYs |
- Sensitivity analysis was only performed for age and stage at diagnosis |
- Sensitivity analysis was only performed for varying life expectancy |
? Uncertain measurements of variabilities in complications and QALYs |
| Directness – reflects the real-life situation? | |||||||
| -Population | ? Characteristics of populations on which complications are based are not described. |
? Characteristics of populations on which complications are based are not described. |
+ | ? Characteristics of populations on which complications are based are not described. |
? Characteristics of populations on which complications are based are not described. |
? Characteristics of populations on which complications are based are not described. |
? Only 35 patients in cohort. |
| -Intervention (DIEP) | ? Characteristics of populations in which intervention is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
+ | ? Characteristics of populations in which intervention is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
? Only 19 patients in cohort |
| -Comparator (implant-based) | ? Characteristics of populations in which comparator is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
- | ? Characteristics of populations in which intervention is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
? Characteristics of populations in which intervention is performed are not described. |
? Only 16 patients in cohort |
| -Time horizon | + | - Only patients with a life expectancy of 7 years after reconstructed included |
? Most women have a longer life-expectancy than 10 years after reconstruction |
? Most women have a longer life-expectancy than 10 years after reconstruction |
+ | ? Most women have a longer life-expectancy than 7 years after reconstruction |
- The effect of breast reconstruction should be measured for a longer period than 12 months |
| -Perspective | + Representative for the US perspective |
+ Representative for the US perspective |
+ Representative for the US perspective |
? A societal perspective is recommended in Dutch guidelines for economic evaluation in healthcare [43]. |
+ Representative for the US perspective |
+ Representative for the US perspective |
+ Representative for the Canadian perspective |
| -Outcomes | ? Characteristics of populations on which outcomes are based is not described. |
? Characteristics of populations on which outcomes are based is not described.. |
? Characteristics of populations on which outcomes are based is not described. |
+ | ? Characteristics of populations on which outcomes are based is not described. |
? Characteristics of populations on which outcomes are based is not described. |
? Outcomes evaluated in only 35 patients |
| Inconsistency | |||||||
| Unexplained variability in results in study. Sensibility used to evaluate if there are inconsistencies. | + |
+ |
+ | + |
+ |
+ |
+ |
| Imprecision | |||||||
| Assessment of how severe the variability is (e.g., range of estimated effects). | + | + | + | + | - Only estimates for age and stage at diagnosis |
- Only estimates for life expectancy |
+ |
| +: No or minor problems; ?: Some problems; -: Major problems. | |||||||
Five studies used previously reported studies to estimate complications and their probabilities. However, it is not stated how the evidence was selected, evaluated, and synthesized to create averages and distributions of the data. A few of the studies, the authors state that they have performed a systematic review to gather the evidence. However, none of studies describes search strategies, inclusion and exclusion of articles, and evaluation of the quality of the included studies and thereby of the input in the model. The two studies that collected data on complications from patients [17,33] did not report how data on complications were collected and how they were defined, and in one of them, the sample size was 35 patients [17]. An inconsistency in complication data in reconstructive surgery is well-known [39] and might have affected the results.
The heterogenicity of included patients introduces a bias, as there are differences between radiated and nonradiated patients, tumor stages, and timing of reconstruction, and length of follow-up that might have implications for cost-effeciency.
In brief, the risk of bias across the studies is high (Table 2). The limitations must be considered serious.
Directness
To assess the directness of the studies, there is a need to evaluate to what extent the populations, interventions, comparators, time horizons, analytic perspectives, and outcomes reflect the real-life situation (Table 2). In most of the included studies, the characteristics of the populations on which interventions, comparators, and outcomes are based are not described in detail, and therefore, the directness of them is unclear. Most of the studies on which complications and outcomes are based have a follow-up of less than 24 months, and therefore, the outcomes and complications in a longer-term perspective are unclear. The relative 10-year survival of breast cancer is 88% [40]. Hence, many women will live with their breast reconstruction for many years, which necessitates reliable long-term outcomes for the health economic evaluation to reflect the real-life situation. Another factor that makes the directness unclear is that most of the modeling studies used a simple decision tree, which only allowed the inclusion of one potential complication or outcome per patient. In a real-life scenario, multiple corrections are common for several breast reconstruction methods [41,42], thus making the directness of the analysis unclear. In brief, the directness across the studies is unclear (Table 2) and could have affected the results.
Inconsistency
Inconsistency concerns unexplained variability in results. Within studies, sensitivity analyses revealed that the greatest uncertainty is complications after DIEP flaps [28,30,33] and life expectancy of the included patients [29,32], which is an expected result. Between studies, the discrepancies in the ICERs can be explained by the fact that the studies used different ‘standard procedures’ as comparison, some of them even ‘mastectomy only’ [29,30,33], and different ways to generate QALYs, and some of them even had a ‘do nothing’ approach as a comparison. Five studies suggested that breast reconstruction with a DIEP flap might be more cost-effective than implant-based breast reconstruction, although implant-based reconstruction could be more cost-effective in patients with a short life-expectancy [28,29]. This is in accordance with the conclusion that implant-based reconstruction is more cost-effective from a 1-year perspective [17]. Moreover, the two studies concluded that implant-based reconstruction is more cost-effective and used generic instruments to evaluate the effectiveness, and little difference could be detected between the methods [17,33], suggesting that the method to evaluate the effectiveness was inadequate. In addition, base-line values were higher in the implant group in one of the studies, indicating that there was a pre-reconstructive difference in quality of life between the groups [17]. In summary, the risk of inconsistencies across studies must be considered low.
Imprecision
Imprecision concerns assessment of how severe the variability is in the studies. All the studies have performed sensitivity analysis with a range of estimates, although two studies only included estimates for age and stage at diagnosis/life expectancy [29,32], and one did not perform any sensitivity analyses at all [17]. The risk of imprecision across studies has to be considered low.
Overall certainty of evidence
The overall certainty of evidence for DIEP-flaps to be cost-effective compared to implant-based breast reconstruction is low (GRADE ƟƟОО). The evidence was downgraded two levels for a high risk of bias and one level for an unclear directness and upgraded one level due to consistency in the results across the studies.
Reporting quality
The quality of reporting was similar across the studies and is presented in Table S2. Notably, few studies included information about whether a health economic analysis plan had been developed and whether it was available anywhere, demographic characteristics of previously published populations that were used as input in the economic models, relevant context information that may have influenced the findings, why the applied perspective was chosen, reasons for discount rates, rationales for the model chosen, methods for analyzing or statistically transform data and for extrapolating data and approaches for validating the model used, estimations of how results vary between subgroups, ethical and equity considerations of the results, and any engagement with stake holders, such as patients (Table S2). Six studies were published before the update of the CHEERS guidelines [27]. Hence, some of the CHEERS items evaluated in this study were not part of the reporting guideline when the included articles were published.
Discussion
This is the first systematic review comparing the cost-effectiveness of DIEP flaps and implant-based breast reconstruction that evaluates the included studies using the GRADE approach.
Cost-effectiveness of DIEP flaps vs. implant-based reconstruction
Among the seven studies meeting the inclusion criteria, five studies suggested that breast reconstruction with a DIEP might be cost-effective compared with implant-based breast reconstruction, especially when a long survival is expected [28,29,32]. Two studies suggesting the opposite used generic quality of life instruments, which are not able to discriminate between different reconstructive techniques [17,33]. Hence, the latter studies can be described more as cost-minimization analyses rather than cost-effectiveness studies. Most elements are adequately reported according to CHEERS.
Nonetheless, a number of scientific weaknesses has been identified in the included studies, and they are discussed later, together with suggestions for future studies in the field.
Methodological issues of the included studies and suggestions for future studies
Perspective
Most of the included studies had a healthcare perspective, and only costs directly related to the healthcare system were included. However, in most countries that have publicly financed welfare systems, direct hospital costs are only part of the available resources consumed when a breast reconstruction is performed. It can therefore be argued that a societal perspective is more adequate when the cost-effectiveness of different breast reconstructive techniques is evaluated. When the primary reconstruction is performed, a DIEP-flap often requires more healthcare resources in terms of time in the operating theater, number of surgeons required per reconstruction, and days in hospital than an implant-based reconstruction, whereas there might be a difference in longevity and amount of maintenance work required between the methods. For example, smaller operations, such as an implant exchange or correction of a capsular contracture, might infer minor costs to the healthcare system and to society as a whole, including loss of production, might be considerable, and therefore of interest when this type of reconstruction is performed. As the long-term survival after breast cancer is high, long-term societal costs have to be considered when the cost-effectiveness of different breast reconstruction techniques is evaluated.
Measurements of benefits with the treatment
In cost-effectiveness studies, generic quality of life instruments are recommended to elicit preference measures [44]. However, for breast reconstruction, these generic instruments are inappropriate as they are not sensitive enough [11], as seen in the included studies [17,33]. The other studies [29,32] used a breast-specific instrument and calculated ‘breast-QALYs’, which is not a validated method. A suggested solution, when generic instruments are too unspecific, is to append ‘bolt-on’ items to generic instruments [44] or to use both disease specific and generic instruments [45]. Another question to ponder is when the health-related quality of life should be measured, and how it should be discounted. It is generally agreed that plastic surgical results cannot be evaluated until 1 year after the surgery, and this could therefore be a miminum follow-up time. Moreover, it has been suggested that changes in satisfaction with the breast and breast-related quality of life might change differently for different reconstructive methods, which also needs to be explored [46]. In brief, to increase the scientific standard of cost-effectiveness studies in breast reconstruction, a standard needs to be established for how QALY-weights should be calculated and when the measurements should be performed.
Usage of comparators
When a cost-effectiveness study is conducted, relevant treatment alternatives have to be compared in order to make sense. In a few of the included studies, a ‘do nothing’-approach, that is mastectomy only [29,30,33], was used as a comparator to different techniques for breast reconstruction. This does not seem to be a relevant comparator as there is a strong norm that women should be offered breast reconstruction post-mastectomy, as stipulated in documents like the Women’s Health and Cancer Rights Act (WHCRA) [47], the New York State (NYS) Breast Cancer Provider Discussion Law [48], and the European Parliament Resolution on breast cancer [49]. Thus, an ICER where mastectomy is compared to breast reconstruction does not seem to be adequate.
Modeling approaches and analysis
The modeling approach must reflect the reconstructive reality, and a model that allows for several complicaitons and corrections is necessary. In some subgroups, both the probability of the treatment and the benefits of it might differ considerably, for example, in patients who have had radiotherapy [50], who experienced surgical complications [51–53], who are obese [54] or have a short life-expectancy [28,29], and between immediate and delayed breast reconstruction [55,56]. This implies that detailed information needs to be included about the patients charachterstics, and that sub analyses might be warranted to answer the question if implant-based or DIEP flap reconstruction is the most cost-effective technique.
Threshold for acceptable costs per QALY
The conclusion regarding whether an internvetion is cost effective or not is dependent on what threshold for acceptable costs per QALY gained the study applies. This threshold generally varies considerably between different healthcare systems. For example, in the US, a threshold of $50,000 to $150,000 is often considered acceptable [57], while a threshold of 20–30,000 GBP per QALY is acceptable in the UK [58]. Many countries do not have a set threshold.
Conclusions
The findings of this present systematic review should be interpreted with caution as the overall certainty of evidence is low (GRADE ƟƟОО). The included studies suggest that DIEP-flaps are cost-effective compared with implant-based breast reconstruction when the applied cost-effectiveness threshold of $50,000 to $100,000 per QALY is used . It is noteworthy that we do not have any high level evidence, regarding cost-effeciency, to support recommendations and decisions in breast reconstruction. According to this review, the following factors can be improved in future studies on this topic: the inclusion of a societal perspective, standardized and validated methods to evaluate benefits, and a modeling approach and analysis that is more compatible with the reconstructive reality. In addition, there is a need for a standardised way to report complications, revisions, time perspective, tumor stage, and oncological treatment, and timing of reconstruction in a standardized fashion to allow for sub-analyses and evaluation of clinical relevance in different groups of patients.
Sources of funding
This study was funded by grants from The Swedish Cancer Society [21 0279 SCIA]. The sources of funding had no role in the design of the study; collection, analysis, and interpretation of data; and in writing the manuscript.
Category
Systematic review.
The synthesis of the data has never previously been reported or presented.
ORCID
Emma Hansson  https://orcid.org/0000-0002-3218-0881
https://orcid.org/0000-0002-3218-0881
References
[1] Giunta RE, Hansson E, Andresen C, et al. ESPRAS survey on breast reconstruction in Europe. Handchir Mikrochir Plast Chir. 2021;53(4):340–348. https://doi.org/10.1055/a-1424-1428
[2] Potter S, Holcombe C, Ward JA, et al. Development of a core outcome set for research and audit studies in reconstructive breast surgery. Br J Surg. 2015;102(11):1360–1371. https://doi.org/10.1002/bjs.9883
[3] Tessler O, Mattos D, Vorstenbosch J, et al. A methodological analysis of the plastic surgery cost-utility literature using established guidelines. Plast Reconstr Surg. 2014;133(4):584e–592e. https://doi.org/10.1097/PRS.0000000000000004
[4] Khajuria A, Prokopenko M, Greenfield M, et al. A meta-analysis of clinical, patient-reported outcomes and cost of DIEP versus implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2019;7(10):e2486. https://doi.org/10.1097/GOX.0000000000002486
[5] Atherton DD, Hills AJ, Moradi P, et al. The economic viability of breast reconstruction in the UK: comparison of a single surgeon’s experience of implant; LD; TRAM and DIEP based reconstructions in 274 patients. J Plast Reconstr Aesthet Surg. 2011;64(6):710–715. https://doi.org/10.1016/j.bjps.2010.11.001
[6] Damen THC, Wei W, Mureau MAM, et al. Medium-term cost analysis of breast reconstructions in a single Dutch centre: a comparison of implants, implants preceded by tissue expansion, LD transpositions and DIEP flaps. J Plast Reconstr Aesthet Surg. 2011;64(8):1043–1055. https://doi.org/10.1016/j.bjps.2010.12.028
[7] Lagares-Borrego A, Gacto-Sanchez P, Infante-Cossio P, et al. A comparison of long-term cost and clinical outcomes between the two-stage sequence expander/prosthesis and autologous deep inferior epigastric flap methods for breast reconstruction in a public hospital. J Plast Reconstr and Aesthet Surg. 2016;69(2):196–205. https://doi.org/10.1016/j.bjps.2015.11.027
[8] Neyt MJ, Blondeel PN, Morrison CM, et al. Comparing the cost of delayed and immediate autologous breast reconstruction in Belgium. Br J Plast Surg. 2005;58(4):493–497. https://doi.org/10.1016/j.bjps.2004.12.002
[9] Palve JS, Luukkaala TH, Kääriäinen MT. Autologous reconstructions are associated with greater overall medium-term care costs than implant-based reconstructions in the Finnish healthcare system: a retrospective interim case-control cohort study. J Plast Reconstr Aesthet Surg. 2022;75(1):85–93. https://doi.org/10.1016/j.bjps.2021.08.020
[10] Tran BNN, Fadayomi A, Lin SJ, et al. Cost analysis of postmastectomy reconstruction: a comparison of two staged implant reconstruction using tissue expander and acellular dermal matrix with abdominal-based perforator free flaps. J Surg Oncol. 2017;116(4):439–447. https://doi.org/10.1002/jso.24692
[11] Phan R, Hunter-Smith DJ, Rozen WM. The use of patient reported outcome measures in assessing patient outcomes when comparing autologous to alloplastic breast reconstruction: a systematic review. Gland Surg. 2019;8(4):452–460. https://doi.org/10.21037/gs.2019.07.04
[12] Eltahir Y, Krabbe-Timmerman IS, Sadok N, et al. Outcome of quality of life for women undergoing autologous versus alloplastic breast reconstruction following mastectomy: a systematic review and meta-analysis. Plast Reconstr Surg. 2020;145(5):1109–1123. https://doi.org/10.1097/PRS.0000000000006720
[13] Toyserkani NM, Jorgensen MG, Tabatabaeifar S, et al. Autologous versus implant-based breast reconstruction: a systematic review and meta-analysis of breast-Q patient-reported outcomes. J Plast Reconstr Aesthet Surg. 2020;73(2):278–285. https://doi.org/10.1016/j.bjps.2019.09.040
[14] Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
[15] Honkanen N, Mustonen L, Kalso E, et al. Breast reconstruction after breast cancer surgery – persistent pain and quality of life 1–8 years after breast reconstruction. Scand J Pain. 2021;21(3):522–529. https://doi.org/10.1515/sjpain-2021-0026
[16] Eltahir Y, Werners L, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132(2):201e–209e. https://doi.org/10.1097/PRS.0b013e31829586a7
[17] Thoma A, Avram R, Dal Cin A, et al. Cost-effectiveness analysis of abdominal-based autogenous tissue and tissue-expander implant following mastectomy. Plast Reconstr Surg Glob Open. 2020;8(10):e2986. https://doi.org/10.1097/GOX.0000000000002986
[18] Kouwenberg CAE, Kranenburg LW, Visser MS, et al. The validity of the EQ-5D-5L in measuring quality of life benefits of breast reconstruction. J Plast Reconstr Aesthet Surg. 2019;72(1):52–61. https://doi.org/10.1016/j.bjps.2018.08.023
[19] Sinno H, Dionisopoulos T, Slavin SA, et al. The utility of outcome studies in plastic surgery. Plast Reconstr Surg Glob Open. 2014;2(7):e189. https://doi.org/10.1097/GOX.0000000000000104
[20] Thoma A, McKnight LL. Quality-adjusted life-year as a surgical outcome measure: a primer for plastic surgeons. Plast Reconstr Surg. 2010;125(4):1279–1287. https://doi.org/10.1097/PRS.0b013e3181d0ae58
[21] Sheckter CC, Matros E, Momeni A. Assessing value in breast reconstruction: a systematic review of cost-effectiveness studies. J Plast Reconstr Aesthet Surg. 2018;71(3):353–365. https://doi.org/10.1016/j.bjps.2017.09.010
[22] Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
[23] Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. https://doi.org/10.1186/2046-4053-4-1
[24] Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66(2):140–150. https://doi.org/10.1016/j.jclinepi.2012.04.012
[25] Brozek JL, Canelo-Aybar C, Akl EA, et al. GRADE guidelines 30: the GRADE approach to assessing the certainty of modeled evidence – an overview in the context of health decision-making. J Clin Epidemiol. 2021;129:138–150. https://doi.org/10.1016/j.jclinepi.2020.09.018
[26] Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook. The GRADE working group. 2013. https://gdt.gradepro.org/app/handbook/handbook.html
[27] Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. https://doi.org/10.1136/bmj-2021-067975
[28] Bloom JA, Shah SA, Long EA, et al. Post-mastectomy tissue expander placement followed by radiation therapy: a cost-effectiveness analysis of staged autologous versus implant-based unilateral reconstruction. Ann Surg Oncol. 2023;30(2): 1075–1083. https://doi.org/10.1245/s10434-022-12619-5
[29] Razdan SN, Cordeiro PG, Albornoz CR, et al. Cost-effectiveness analysis of breast reconstruction options in the setting of postmastectomy radiotherapy using the BREAST-Q. Plast Reconstr Surg. 2016;137(3):510e–517e. https://doi.org/10.1097/01.prs.0000479935.92904.a3
[30] Grover R, Padula WV, Van Vliet M, et al. Comparing five alternative methods of breast reconstruction surgery: a cost-effectiveness analysis. Plast Reconstr Surg. 2013;132(5):709e–723e. https://doi.org/10.1097/PRS.0b013e3182a48b10
[31] Klifto KM, Tecce MG, Serletti JM, et al. Comparison of nine methods of immediate breast reconstruction after resection of localized breast cancer: a cost-effectiveness Markov decision analysis of prospective studies. Microsurgery. 2022;42(5): 401–427. https://doi.org/10.1002/micr.30882
[32] Matros E, Albornoz CR, Razdan SN, et al. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135(4):937–946. https://doi.org/10.1097/PRS.0000000000001134
[33] Kouwenberg CAE, Mureau MAM, Kranenburg LW, et al. Cost-utility analysis of four common surgical treatment pathways for breast cancer. Eur J Surg Oncol. 2021;47(6):1299–1308. https://doi.org/10.1016/j.ejso.2020.11.130
[34] Thoma A, Avram R, Dal Cin A, et al. Comparing the clinical and cost-effectiveness of abdominal-based autogenous tissue and tissue-expander implant: a feasibility study. Plast Reconstr Surg Glob Open. 2020;8(10):e3179. https://doi.org/10.1097/GOX.0000000000003179
[35] Klifto KM, Christopher A, Morris M, et al. Cost-effectiveness of nine methods of immediate breast reconstruction for women with localized breast cancer not receiving radiation therapy: a Markov/Monte Carlo analysis. J Am Coll Surg. 2021;233(5):s34. https://doi.org/10.1016/j.jamcollsurg.2021.07.045
[36] Kouwenberg CAE, de Ligt KM, Kranenburg LW, et al. Long-term health-related quality of life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast Reconstr Surg. 2020;146(1):1–13. https://doi.org/10.1097/PRS.0000000000006887
[37] Hansson E, Sandman L, Davidson T. A systematic review of direct preference measurements in health states treated with plastic surgery. J Plast Surg Hand Surg. 2022;56(3):180–190. https://doi.org/10.1080/2000656X.2021.1953039
[38] Brorson F, Elander A, Thorarinsson A, et al. Patient reported outcome and quality of life after delayed breast reconstruction – an RCT comparing different reconstructive methods in radiated and non-radiated patients. Clin Breast Cancer. 2022;22(8): 753–761. https://doi.org/10.1016/j.clbc.2022.09.004
[39] Parikh RP, Sharma K, Qureshi AA, et al. Quality of surgical outcomes reporting in plastic surgery: a 15-year analysis of complication data. Plast Reconstr Surg. 2018;141(6):1332–1340. https://doi.org/10.1097/PRS.0000000000004362
[40] Åhlin E (ed). Cancer i siffror 2023. The Swedish National Board of Social Affairs and Health Care and The Swedish Cancer Association. 2023. Stockholm. https://static-files.cancerfonden.se/Cancer-i-siffror-2023.pdf
[41] Levine SM, Lester ME, Fontenot B, et al. Perforator flap breast reconstruction after unsatisfactory implant reconstruction. Ann Plast Surg. 2011;66(5):513–517. https://doi.org/10.1097/SAP.0b013e3182012597
[42] Roostaeian J, Yoon AP, Ordon S, et al. Impact of prior tissue expander/implant on postmastectomy free flap breast reconstruction. Plast Reconstr Surg. 2016;137(4):1083–1091. https://doi.org/10.1097/01.prs.0000481044.61991.6b
[43] Versteegh M, Knies S, Brouwer W. From good to better: new Dutch guidelines for economic evaluations in healthcare. Pharmacoeconomics. 2016;34(11):1071–1074. https://doi.org/10.1007/s40273-016-0431-y
[44] Longworth L, Yang Y, Young T, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18(9):1–224. https://doi.org/10.3310/hta18090
[45] Whittal A, Meregaglia M, Nicod E. The use of patient-reported outcome measures in rare diseases and implications for health technology assessment. Patient. 2021;14(5):485–503. https://doi.org/10.1007/s40271-020-00493-w
[46] Sadok N, Refaee MS, Eltahir Y, et al. Quality of life 9 to 13 years after autologous or alloplastic breast reconstruction: which breast remains best? Plast Reconstr Surg. 2023;151(3):467–476. https://doi.org/10.1097/PRS.0000000000009899
[47] U.S. Department of Labour. The Women’s Health and Cancer Rights Act (WHCRA). Whashington DC, USA; 1998. https://www.dol.gov/sites/default/files/ebsa/about-ebsa/our-activities/resource-center/publications/cagwhcra.pdf
[48] The New York State Senate. The New York State (NYS) Breast Cancer Provider Discussion Law. Bill S.6993-B. New York, USA; 2010. https://legislation.nysenate.gov/pdf/bills/2009/S6993B
[49] The European Parliament. European Parliament resolution on breast cancer in the European Union (2002/2279(INI)). Brussels, Belgium; 2002. http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+TA+P5-TA-2003-0270+0+DOC+XML+V0//EN
[50] Endara MR, Verma K, Nahabedian MY. Tertiary breast reconstruction using a free contralateral latissimus dorsi musculocutaneous flap. J Reconstr Microsurg. 2014;30(2):141–143. https://doi.org/10.1055/s-0033-1354743
[51] Higgins KS, Gillis J, Williams JG, et al. Women’s experiences with flap failure after autologous breast reconstruction: a qualitative analysis. Ann Plast Surg. 2017;78(5):521–525. https://doi.org/10.1097/SAP.0000000000000910
[52] Mahoney B, Walklet E, Bradley E, et al. Experiences of implant loss after immediate implant-based breast reconstruction: qualitative study. BJS Open. 2020;4(3):380–390. https://doi.org/10.1002/bjs5.50275
[53] Weick L, Ericson A, Sandman L, et al. Patient experience of implant loss after immediate breast reconstruction: an interpretative phenomenological analysis. Health Care Women Int. 2023;44(1):61–79. https://doi.org/10.1080/07399332.2021.1944152
[54] Fischer JP, Nelson JA, Sieber B, et al. Free tissue transfer in the obese patient: an outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast Reconstr Surg. 2013;131(5):681e–692e. https://doi.org/10.1097/PRS.0b013e31828e2159
[55] Yoon AP, Qi J, Brown DL, et al. Outcomes of immediate versus delayed breast reconstruction: results of a multicenter prospective study. Breast. 2018;37:72–79. https://doi.org/10.1016/j.breast.2017.10.009
[56] D’Souza N, Darmanin G, Fedorowicz Z. Immediate versus delayed reconstruction following surgery for breast cancer. Cochrane Database Syst Rev. 2011;(7):CD008674. https://doi.org/10.1002/14651858.CD008674.pub2
[57] Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Exp Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178. https://doi.org/10.1586/14737167.8.2.165
[58] Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ. 2007;335(7616):358–359. https://doi.org/10.1136/bmj.39308.560069.BE