ORIGINAL RESEARCH ARTICLE
Limited debridement combined with ReCell® Techniques for deep second-degree burns
Yue Zhang, Kai Guo, Chenyang Tian, Ling Tong, Dahai Hu and Yunchuan Wang
Department of Burns and Cutaneous Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China
ABSTRACT
Background: The purpose of this article is to introduce a method that combines limited debridement and ReCell® autologous cell regeneration techniques for the treatment of deep second-degree burn wounds.
Method: A total of 20 patients suffered with deep second-degree burns less than 10% of total body surface area (TBSA) who were admitted to our department, from June 2019 to June 2021, participated in this study. These patients first underwent limited debridement with an electric/pneumatic dermatome, followed by the ReCell® technique for secondary wounds. Routine treatment was applied to prevent scarring after the wound healed. Clinical outcomes were scored using the Vancouver Scar Scale (VSS).
Results: All wounds of the patients healed completely. One patient developed an infection in the skin graft area and finally recovered by routine dressing changes. The average healing time was 12 days (range: 10–15 days). The new skin in the treated area was soft and matched the colour of the surrounding normal skin and the VSS score ranged from 3~5 for each patient. Of the 20 patients, 19 were very satisfied and 1 was satisfied.
Conclusions: This article reports a useful treatment method that combines electric dermatome-dependent limited debridement and the ReCell® technique for the treatment of deep second-degree burn wounds. It is a feasible and effective strategy that is easy to implement and minimally invasive, and it is associated with a short healing time, mild scar formation and little damage to the donor skin area.
KEYWORDS: Limited debridement; electric dermatome; ReCell® Autologous cell regeneration techniques; deep second-degree burn; wound healing
Citation: Journal of Plastic Surgery and Hand Surgery 2024; 59: 72–76. DOI: https://doi.org/10.2340/jphs.v59.24557.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 30 October 2023; Accepted: Jan 22, 2024; Published: 20 May 2024.
CONTACT Dahai Hu and Yunchuan Wang hudhai@fmmu.edu.cn; wangyunchuan@fmmu.edu.cn Department of Burns and Cutaneous Surgery, Xijing Hospital, Fourth Military Medical University, 127 Changle West Road, Xi’an, Shaanxi 710032, China
Competing interests and funding: The authors declare that they have no competing interests.
Introduction
Deep second-degree burns are injuries that affect the epidermis and part of the dermis [1]. The methods that are chosen for treating deep second-degree burns have important effects on the outcome of the injury, and the choice of treatment has always been difficult for clinicians. Conservative treatment and wound skin grafting are commonly used clinically [2]. Conservative treatment requires multiple dressing changes, which increases not only the pain but also the treatment time. As a result, the wound surface is susceptible to infection, resulting in severe scarring after wound healing and thus negatively affecting the appearance and physiological function of the area [3]. However, early escharotomy with medium-thickness skin grafting causes greater damage to the donor site and involves the introduction of new wounds and the formation of scars. Additionally, noticeable linear scars often appear at the junction of graft skins, which also affects the appearance and quality of life of patients. Furthermore, it is difficult to control the wound depth during tangential excision; this can lead to incomplete removal of necrotic tissues, which decreases the survival rate of skin grafts, or result in damage to some active skin tissues and skin appendages due to excessive debridement, rendering the wound more difficult to heal [4]. Therefore, it is important to identify a method that can accurately remove necrotic tissue from the wound while preserving healthy or partially degenerated tissue as much as possible. Thus, limited debridement can effectively reduce the local inflammatory response and microcirculation stasis and accelerate the angiogenesis and self-healing of the wound, thus promoting the healing of burns. An electric/pneumatic dermatome can precisely control the thickness of the skin during the excision progress and has been used during the debridement of deep second-degree burns; this approach removes necrotic tissue and preserves as much of the dermal tissue and skin appendages as possible, which is conducive to wound healing.
In addition, if too few epidermal cells remain, it will seriously hinder the re-epithelialisation of deep second-degree burn wounds. Autologous skin grafting can rapidly heal deep second-degree burn wounds by providing epithelial tissue for the wound surface. Increasing the number of autologous epidermal cells on the wound surface has a similar effect on accelerating wound healing. ReCell® autologous cell regeneration techniques are methods that use the ReCell device to separate collected samples of autologous split-thickness skin and generate a cell suspension that can be transplanted onto the wound [5]. The advantage of the ReCell technique over conventional skin grafting techniques is that it increases the number of autologous epidermal cells but causes only very little damage to the patient’s donor site because this approach can achieve an expansion ratio of 1:40 or even 1:80 [6]. In this study, limited debridement was performed on deep second-degree burn wounds to provide a good basis for the healing process. Then, a small amount of autologous epidermal tissue was collected and separated to generate a cell suspension, which was sprayed onto the prepared wound surface before bandaging [7,8]. The dressing was changed 3 days after the surgery, but the inner layer of dressing close to the wound was not replaced. Most of the wound surface was epithelialized and healed within 10–15 days, which is a substantially shortened time for burn wound healing. This study provides a reliable, effective method for the treatment of deep second-degree burns.
Objects and methods
Patient selection
From June 2019 to June 2021, our department admitted 247 patients with deep second-degree burns, of which 20 patients were identified and included in this study. The inclusion criteria were as follows: (1) the patient was first admitted to our hospital within 24 h after injury; (2) the patient suffered epidermal and partial dermal damage, which is characteristic of deep second-degree burns; (3) the burn area varied from 1% to 10% of the patient’s TBSA; (4) the cause of injury was flame or boiling water and (5) the patient was between 18 and 60 years old (Table 1). All the patients signed informed consent according to the requirements of the Ethics Committee of our Hospital. During the study period, an electric/pneumatic dermatome was used for limited debridement, which was combined with the ReCell® technique to repair deep second-degree burn wounds. The exclusion criteria for this study were as follows: (1) the patient suffered superficial partial-thickness burns or full thickness burns; (2) the patient suffered chemical burns or had burn areas over 10% or below 1% of the TBSA; (3) the patients had systemic disease, such as diabetes, sepsis, and mental disorders; and (4) the patient was a long-term smoker or exhibited low compliance. Table 1 presents the patients’ data.
Surgical method
After a patient was admitted to the hospital, the wound was immediately treated with wet cold dressings for 30 min. The wound was then rinsed with saline and wrapped with traditional dressings. The degree of the burn was assessed, and the patients’ injury history was collected. The included patients were scheduled for surgery after the relevant examinations were finished. Communication with the patient and his or her family members was required 1 day before surgery to confirm the donor area, which was usually the scalp or outer thigh. The hair around the wound was shaved for disinfection.
On the day of the operation, routine disinfection and surgical draping were arranged in turn after anaesthesia. The wound surface was rinsed repeatedly with normal saline. Subsequently, an electric/pneumatic dermatome (thickness was set to 0.1 mm; Zimmer8821 USA) was used for the limited debridement of the deep second-degree burns. A blade with an appropriate width was selected according to the size of the wound surface. The excision depth was controlled within the epidermis and superficial dermis. The superficial necrotic tissue was repeatedly removed until needle-point bleeding points or redness appeared on the wound surface. Part of the parabiotic tissue on the wound was preserved. After complete haemostasis, the wound was covered with saline-wetted gauze for later ReCell skin grafting. The ReCell device (Avita Medical, California, USA) was opened and used according to the instructions. The skin extraction range was reserved according to the wound size with an expansion rate of 1:80 for the preparation of the cell suspension. The thickness of the skin graft from the donor area was approximately 0.2~0.3 mm. After the cell suspension was sprayed on the wound with a flat-head syringe, the surgical area was covered with a protective film from the ReCell service, and the outer layer was bandaged with conventional dressings. Postoperative elastic bandages were then used to further immobilize the surgical area to enhance the bonding of the skin graft. No other treatments were required after the surgery. On the 3rd day after the operation, the surgical area was opened, and the dressing was changed until the wound healed. Then, drugs to prevent scarring and pressure clothing were routinely used. If necessary, functional exercises were performed to assist the recovery of injured limbs.
Observing targets
Photographs were taken with a Nikon camera in natural light before and after treatment. Complications of the operation, such as odour, blood, secretions, and infection, were observed. The wound healing time was recorded. The appearance, pigmentation, and scarring of the donor and recipient sites, as well as patient satisfaction with these sites, were also observed during follow-up. All the patients were followed up for at least 3 months (range: 3–12 months; mean: 6 months). The VSS was used to evaluate the colour, thickness, vascular distribution, and softness of the treated area 3 months after surgery. On the VSS scale, the highest score was 18, and the lowest was 0. A higher score indicated a more severe scar.
Statistical analysis
All the data are presented as the mean ± standard error of the mean; SPSS 18.0 and GraphPad Software were used for statistical analysis.
Results
In this study, the wounds of all 20 patients were completely healed. The new skin was intact without wrinkles and had good blood circulation with the surrounding skin. One of the patients developed an infection in the skin graft area and finally recovered after five dressing changes. The average healing time was 12 days (range: 10~15 days). The 3-month follow-up after the operation showed that the appearance of the skin graft area was good and that the graft had good elasticity, and there was no obvious scar hyperplasia. The patient VSS scores ranged from 3 to 5 points (Table 2). Among the 20 patients, 19 were very satisfied with the appearance and function of the wound surface and the donor sites, 1 was satisfied, and no patients were dissatisfied (Figure 1).
| Number of cases | Colour | Vascular distribution | Softness | Thickness |
| 20 | 1.36 ± 0.11 | 0.32 ± 0.14 | 1.25 ± 0.51 | 1.45 ± 0.24 |
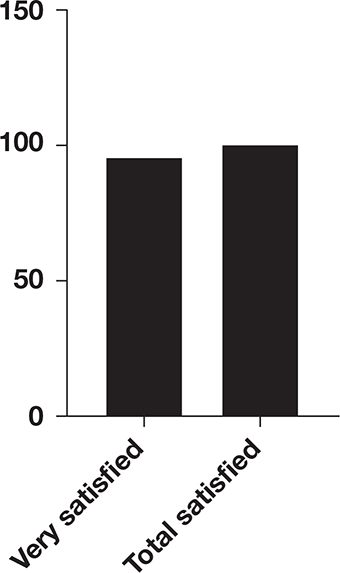
Figure 1. Patients’ satisfaction.
The overall satisfaction rate was 100% (20/20). The detailed results are as follows.
Representative cases
Case one
A 38-year-old man accidentally scalded his left hand with hot oil at work, resulting in a deep second-degree burn, and the appearance of approximately 1% of the wound surface was red and white (Figure 2A). The patient was admitted to the hospital 6 h after the injury, and then, the wound was routinely rinsed with cold water for 30 min and bandaged with vaseline gauze. Next, related examinations were conducted. On the third day after the injury, the patient’s wound on his left hand was treated with limited debridement and the ReCell® technique under anaesthesia in the operating room. An electric dermatome was used to debride the white area near the junction of the thumb and the index finger on the back of the left hand. The thickness of the dermatome was set to 0.1 mm and a blade with a width of 5 cm was selected to remove the superficial necrotic tissue. Debridement was stopped when scattered bleeding points appeared on the wound base. Most of the parabiotic tissue was preserved and then haemostasis was performed. A 1 cm × 1 cm split-thickness skin graft was taken from the head, and a cell suspension was generated using a ReCell service; then, the cell suspension was sprayed on the prepared wound (Figure 2B).
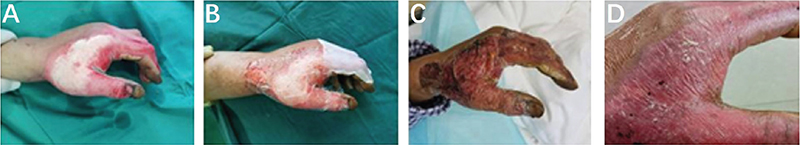
Figure 2. (A) The left hand was scalded by hot oil, resulting in a deep second-degree burn. (B) Limited debridement and ReCell technique were performed on the left hand. (C) Wound surface on the 3rd day after surgery. (D) Wound surface on the 10th day after surgery. The dressing was removed on the 3rd day after the surgery, and the wound on the left hand was dry (C). The wound healed 10 days after the surgery (D). The patient was very satisfied with the appearance and function of the area treated with surgery.
Case two
A 54-year-old woman accidentally scalded her right upper limb with boiling water, resulting in mixed burns on approximately 4% of the wound surface (Figure 3A). Surgery was performed on the third day after the injury. Limited debridement combined with the ReCell® technique was performed on a 10 cm × 10 cm deep second region on the lateral right upper limb. A tool bit with a width of 10 cm was selected. After the necrotic skin tissue (0.2 mm thickness) was removed with an electric scalpel, intensive needle-like haemorrhagic points emerged. Debridement was stopped and haemostasis was performed. A 2 cm × 2 cm area on the outer side of the right thigh was selected for split-thickness skin extraction, and ReCell grafting was performed. The wound on the rest of the right upper limb was treated with dressing changes (Figure 3B). The dressing was removed on the 3rd day after the operation, and the wound base on the right upper limb was dry, and no secretions were observed (Figure 3C). The wound was healed on the 11th day after the surgery (Figure 3D). The patient was very satisfied with the wound healing process.
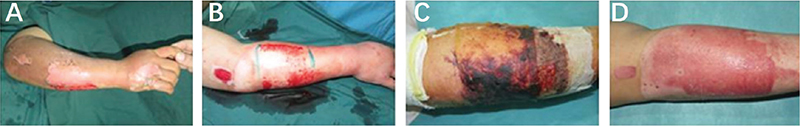
Figure 3. (A) Preoperative photograph of the right upper limb. (B) The area of the deep second region on the lateral right upper limb. (C) Wound surface on the 3rd day after surgery. (D) The wound healed well 11 days after surgery.
Discussion
With the improvement of burn healing rate and the development of plastic surgery technologies, the goal of burn treatment has shifted from wound healing to reducing scars and improving quality of life [9]. For burns and scald wounds, if healing takes more than 3 weeks, severe scars often form [10]. For deep second-degree burns, the average time required for healing with conservative treatment is 3 weeks or longer. If skin grafting is performed after several dressing changes, there is a possibility of exacerbated wound infection and difficulty in healing [11]. At present, these wounds are generally repaired by the early excision of necrotic tissue and transplantation of skin. Nevertheless, this method often deepens wounds and causes secondary damage to the donor site, and the effectiveness of skin grafting also varies among individual patients. Superficial necrotic tissue in deep second-degree burns does not easily fall off and is easily infected. Due to the dysfunction of microcirculation, the inflammatory response, peroxidative damage and other mechanisms, progressive deepening often occurs in the early stages after a deep second-degree burn injury [12]. Additionally, the epidermis and some skin appendages are damaged and difficult to self-healing [13]. Optimising surgical plans and shortening the wound healing time are of great significance for recovery.
Deep second-degree burn wounds include a necrotic region, stasis region and hyperemic region; the transformation of the stasis region to normal tissue is critical in the process of wound repair [14]. Clinicians usually accelerate wound healing by excising the necrotic tissue to clean the necrosis area, and promoting recovery of stasis area. However, in previous treatment methods, during the process of excising eschars with roller knives or blades, the depth is hard to control [15]. Consequently, incomplete removal of necrotic tissues will probably occur. If debridement excessively, the stasis area or even the hyperaemia area might be damaged, which will lead to exacerbated wounds, thereby affecting wound healing [16,17]. Our study proposes a limited debridement technique that uses an electric/pneumatic dermatome to perform controlled debridement according to the depth of the wound surface. By adjusting the scale at the end of the handle, the thinnest wound eschar with a thickness of 0.1 mm could be obtained. This method can be more precisely controlled and could accurately remove the necrotic area and preserve the stasis area and hyperaemia area as much as possible. Although this approach cannot reverse the necrosis of the burned tissue, it can remove the new inflammatory granulation tissue, significantly reduce the local inflammatory response, and prevent bacterial colonisation and cross-contamination. In addition, this approach can prevent the wound from deepening and, to a great extent, preserve the remaining hair follicles, sweat glands and normal tissues, thereby promoting wound healing. Attention should be given to the depth of the debridement so that the parabiotic tissue is preserved because the wound cannot heal itself if the residual dermis is removed.
The self-healing of wounds is achieved through the migration of keratinocytes from the wound edge. If too few residual epidermal cells are present, it will seriously hinder the process of re-epithelialisation of deep second-degree wounds. Increasing the number of autologous epidermal cells on the wound has a similar effect of accelerating wound healing. The ReCell® technique was developed by Australian dermatologists in 1992 and applied in the medical field. In many European countries, this technique has been introduced into the routine clinical treatment of skin lesions, such as skin trauma, burns, scar repair and abnormal pigmentation [18–20]. This method can rapidly increase the number of autologous epidermal cells by collecting cells from the dermal-epidermal junction of skin and delivering them to the wound as a cell suspension. In this method, a cell suspension that has a high expansion ratio and an amplification efficiency of approximately 1:80 can be prepared from a small amount of donor tissue. This greatly reduces the likelihood of damage to the donor site and the formation of scars while improving the quality of wound repair [21,22]. The wound itself is used as a tissue culture system to reproduce keratinocytes, melanocytes and papillary fibroblasts, and the cells migrate to regenerate the epidermis, matching the colour and texture of the surrounding skin. The ReCell® technique can provide sufficient autologous epidermal cells for patients with deep second-degree burns. Compared with conventional skin grafting, the ReCell® technique reduces the damage caused to the donor site and prevents the formation of scars at the donor site; thus, it may be a good choice for deep second-degree burn patients [23,24]. Nonetheless, studies on the application of this technique in the treatment of deep second-degree burns are very limited [25].
In this study, 20 patients with deep second-degree burns were treated with limited debridement and the ReCell® technique. An electric/pneumatic dermatome was used to establish a favourable wound environment, and the number of autogenous epidermal cells to be used for transplantation was increased by ReCell. This clinical method reduced the average wound healing time to 12 days and minimized the damage caused to the donor site. The new skin graft was intact without wrinkles and had good blood circulation with the surrounding skin. Only one patient suffered an infection of the surgical area, but it was resolved after five dressing changes. Follow-up examination showed that the skin in the grafting area had a good appearance, good elasticity, and soft texture, and the colour was close to that of the surrounding normal skin. Furthermore, the VSS scores were ideal, and patient’s satisfaction was high. In conclusion, in the treatment of deep second-degree burn wounds, the proposed method (limited debridement combined with the ReCell® technique) can maximize the preservation of parabiotic tissue and achieve rapid epithelialisation. Moreover, the cells need not be cultured and thus can be prepared quickly, which greatly improves the quality of wound repair and reduces long-term scar formation.
Our clinical results are consistent with the results of treating acne scars with the ReCell® technique, as reported by Dr Qiao [26]. The similarity is that the injured sites in both patient populations retain part of their dermal structure. ReCell is suitable for skin injuries with partial dermal injury and has satisfactory clinical results and patient feedback. The key to the success of this treatment method is to select appropriate patients, and the depth of the patient’s injury must be precisely assessed to ensure that the patient has a residual dermal structure. The maximum area that can be treated with the ReCell® technique is 320 cm2, and the thickness is 0.15~0.2 mm. Only with a full understanding of its application principles and surgical methods can it provide more refined, specialized clinical guidance and treatment plans to patients. Postoperative elastic bandages are used to compress the surgical area to ensure that the skin grafts are fixed and closely adhered to the wound surface, providing good conditions for the skin grafts [27].
There are also some limitations to our study, such as a lack of quality control and incomplete tissue utilisation. In addition, more basic, mechanism-related samples and detailed studies are needed in the future.
Conclusion
The proposed treatment method, limited debridement combined with ReCell® autologous cell regeneration techniques, provides a feasible and effective method for the treatment of deep second-degree burn wounds. This method has the advantages of a simple operation, quick healing time, mild scar formation and little damage at the donor skin site, and this method is worthy of further study and promotion. However, it is important to fully assess the burn depth before surgery because the ReCell® technique can only be used when part of the dermis structure remains.
Acknowledgements
The authors would like thank Prof. Dahai Hu and Prof. Yunchuan Wang for helpful suggestions. This study was supported by National Natural Science Foundation of China (No. 82172210).
All procedures were approved by the Ethics Committee of our university.
The data and materials used to support the findings of this study are available from the corresponding author upon request.
References
1. Honnegowda TM, Kumar P, Udupa P, et al. Epidemiological study of burn patients hospitalised at a burns centre, Manipal. Int Wound J. 2019; 16(1): 79–83. https://doi.org/10.1111/iwj.12995
2. Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. 2009; 36(4): 583–596. https://doi.org/10.1016/j.cps.2009.05.001
3. Oryan A, Alemzadeh E, Moshiri A. Burn wound healing: present concepts, treatment strategies and future directions. J Wound Care. 2017; 26(1): 5–19. https://doi.org/10.12968/jowc.2017.26.1.5
4. Atkin L. Understanding methods of wound debridement. Br J Nurs. 2014; 23(12): S10–S12, S14–S15. https://doi.org/10.12968/bjon.2014.23.sup12.S10
5. Yu PX, Diao W, Qi Z, et al. Effect of dermabrasion and ReCell® on large superficial facial scars caused by Burn, Trauma and Acnes (big up tri, open). Chin Med Sci J. 2016; 31(3): 173–179. https://doi.org/10.1016/S1001-9294(16)30047-5
6. Wood FM, Giles N, Stevenson A, et al. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. 2012; 38(1): 44–51. https://doi.org/10.1016/j.burns.2011.03.001
7. Moris V, Cristofari S, Stivala A, et al. Recell in post-traumatic cases: preliminary results. J Plast Reconstr Aesthet Surg. 2020; 73(10): 1897–1916. https://doi.org/10.1016/j.bjps.2020.03.009
8. Holmes JT, Molnar JA, Shupp JW, et al. Demonstration of the safety and effectiveness of the RECELL((R)) System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns. 2019; 45(4): 772–782. https://doi.org/10.1016/j.burns.2018.11.002
9. Tenenhaus M, Rennekampff HO. Surgical advances in burn and reconstructive plastic surgery: new and emerging technologies. Clin Plast Surg. 2012; 39(4): 435–443. https://doi.org/10.1016/j.cps.2012.07.012
10. Litt JS. Evaluation and management of the burn patient: a case study and review. Mo Med. 2018; 115(5): 443–446.
11. Coban YK. Infection control in severely burned patients. World J Crit Care Med. 2012; 1(4): 94–101. https://doi.org/10.5492/wjccm.v1.i4.94
12. Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018; 123: 3–17. https://doi.org/10.1016/j.addr.2017.09.018
13. Magne B, Lataillade JJ, Trouillas M. Mesenchymal stromal cell preconditioning: the next step toward a customized treatment for severe burn. Stem Cells Dev. 2018; 27(20): 1385–1405. https://doi.org/10.1089/scd.2018.0094
14. Hall C, Hardin C, Corkins CJ, et al. Pathophysiologic mechanisms and current treatments for cutaneous sequelae of burn wounds. Compr Physiol. 2017; 8(1): 371–405. https://doi.org/10.1002/cphy.c170016
15. Smith F, Dryburgh N, Donaldson J, et al. Debridement for surgical wounds. Cochrane Database Syst Rev. 2011; 5: CD006214. https://doi.org/10.1002/14651858.CD006214.pub3
16. Girard D, Laverdet B, Buhé V, et al. Biotechnological management of skin burn injuries: challenges and perspectives in wound Healing and sensory recovery. Tissue Eng Part B Rev. 2017; 23(1): 59–82. https://doi.org/10.1089/ten.teb.2016.0195
17. Nazarko L. Advances in wound debridement techniques. Br J Community Nurs. 2015; Suppl Community Wound Care): S6, S8. https://doi.org/10.12968/bjcn.2015.20.Sup6.S6
18. Sood R, Roggy DE, Zieger MJ, et al. A comparative study of spray keratinocytes and autologous meshed split-thickness skin graft in the treatment of acute burn injuries. Wounds 2015; 27(2): 31–40.
19. Cooper-Jones B, Visintini S. A Noncultured Autologous Skin Cell Spray Graft for the Treatment of Burns. 2018 Mar 1. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016–2021. 168.
20. Hu Z, Guo D, Liu P, et al. Randomized clinical trial of autologous skin cell suspension for accelerating re-epithelialization of split-thickness donor sites. Br J Surg. 2017; 104(7): 836–842. https://doi.org/10.1002/bjs.10508
21. Larson KW, Austin, CL, Thompson SJ. Treatment of a full-thickness burn injury with NovoSorb biodegradable temporizing matrix and RECELL autologous skin cell suspension: a case series. J Burn Care Res. 2020; 41(1): 215–219. https://doi.org/10.1093/jbcr/irz179
22. Wu CJ, Li L-J, Liao W-C, et al. Using various skin graft techniques in major burn reconstruction: a lesson learned from a Taiwanese Cornstarch explosion. Ann Plast Surg. 2021; 86(2 Suppl 1): S30–S34. https://doi.org/10.1097/SAP.0000000000002705
23. Singh M, Nuutila K, Kruse C, et al. Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast Reconstr Surg. 2015; 136(4): 524e–530e. https://doi.org/10.1097/PRS.0000000000001634
24. Ren J, Liu J, Yu N, et al. The use of noncultured regenerative epithelial suspension for improving skin color and scars: a report of 8 cases and review of the literature. J Cosmetic Dermatol. 2019; 18(5): 1487–1494. https://doi.org/10.1111/jocd.13071
25. Holmes IJ, Molnar JA, Carter JE, et al. A comparative study of the ReCell® device and autologous spit-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018; 39(5): 694–702. https://doi.org/10.1093/jbcr/iry029
26. Chen Q, Yu N, Liu Z, et al. The clinical efficacy of ReCell® autologous cell regeneration techniques combined with dermabrasion treatment in Acne Scars. Aesthetic Plast Surg. 2020; 44(2): 535–542. https://doi.org/10.1007/s00266-019-01481-8
27. Faure C. Negative pressure treatment devices. Soins. 2014; 782: 30–32. https://doi.org/10.1016/j.soin.2013.12.006