ORIGINAL RESEARCH ARTICLE
Finnish translation and linguistic validation of the FACE-Q Head and Neck Cancer Module
Lotta Varakas, MBa, Ian Barner-Rasmussen, PhDb, Aaro Haapaniemi, PhDc, Andrew Lindford, PhDb, Patrik Lassus, PhDb and Pauliina Homsy, PhDb
aDepartment of Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; bDepartment of Plastic Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; cDepartment of Otorhinolaryngology – Head and Neck Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
ABSTRACT
Head and neck cancer (HNC) and its treatment can result in permanent changes to a patient’s appearance, speaking, eating, and psychosocial well-being. To better assess the impact of the disease on HNC patients, the FACE-Q Head and Neck Cancer Module, a health-related quality-of-life instrument, was developed. The aim of this study was to produce and linguistically validate a Finnish version of the module. The module was translated into Finnish following the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines. A total of 51 patients who had undergone tumour resection and reconstruction of the oral cavity, tonsil, or tongue area between 2019 and 2021 were approached for a pilot study. They completed the translated module and provided feedback on any linguistic issues. Adjustments were made based on the pilot study comments. The FACE-Q Head and Neck Cancer Module translated well into Finnish. Twenty-one (41%) patients participated in the survey, 12 men (57%) and nine women (43%) with a median age of 66 years (range 48–89 years). The median time since surgery was 3 years (range 1–4 years). Based on the feedback from the pilot study participants, one word was changed, and one question was rewritten. Otherwise, no deficiencies were identified in the language of the module. In summary, this study produced a linguistically valid Finnish version of the FACE-Q Head and Neck Cancer Module, enabling its application in evaluating the health-related quality-of-life among Finnish HNC patients who have undergone reconstructive surgery.
KEYWORDS: Head and neck cancer; health-related quality of life; patient-reported outcomes; FACE-Q; FACE-Q Head and Neck Cancer Module
Citation: Journal of Plastic Surgery and Hand Surgery 2024; 59: 141–145. DOI: https://doi.org/10.2340/jphs.v59.40518.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 6 April 2024; Accepted: 2 October 2024; Published: 6 November 2024
CONTACT Pauliina Homsy Pauliina.homsy@hus.fi Department of Plastic Surgery, University of Helsinki and Helsinki University Hospital, Puistosairaala, P.O. Box 281, 00029 HUS, Helsinki, Finland
Competing interests and funding: The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
This research was supported by funding from the Helsinki University Musculoskeletal and Plastic Surgery Research Center.
Introduction
Cancer located in the head and neck area is the seventh most common type of cancer globally [1–3]. Treatment of head and neck cancer (HNC) may involve tumour resection and reconstructive surgery of the surgical defect, frequently combined with non-surgical treatment, such as radiotherapy or chemotherapy [1, 4, 5]. Since this area contains several structures responsible for critical functions, such as speaking, swallowing, eating, and socialising, HNC and its treatments can disrupt these functions, resulting in challenges in daily activities [6–9]. Additionally, the impact of the treatment on aesthetically important structures as well as the psychosocial well-being can ultimately affect the patient’s overall quality of life [6, 7, 10–12].
Several patient-reported outcome measures (PROMs) exist for the assessment of health-related quality of life in HNC patient’s post-treatment, including the EORTC H&N35 and the UW-QOL [1, 11, 13, 14]. However, many of these surveys do not capture all the aspects relevant to HNC patients. In particular, several of the available PROMs focus on changes in functionality, such as swallowing and eating, but only a few address psychosocial aspects or appearance, both of which can have a significant impact on a patient’s quality of life [13, 15, 16]. Furthermore, several measures were developed without any patient input, putting them at a disadvantage regarding the relevance and validity of their questions [13, 15].
To address the need for a more holistic PROM for HNC patients, the FACE-Q Head and Neck Cancer Module was developed [13]. It is a PROM specifically designed for this patient group and has currently been translated into seven languages, enabling the comparison of data between countries [17–19]. The questionnaire is divided into four domains covering facial appearance, facial functionality, psychosocial distress, and experience of care. It consists of 14 different scales, which can be used separately or together, allowing the selection of only relevant scales for each patient or research question [13]. This study aimed to produce and linguistically validate a Finnish version of the FACE-Q Head and Neck Cancer Module.
Materials and methods
Translation of the FACE-Q Head and Neck Cancer Module
The FACE-Q Head and Neck Cancer Module was translated into Finnish following the guidelines established by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and in accordance with the Mapi Research Trust guidelines [20, 21]. Permission to translate the module was obtained from the license holder [17]. The translation process consisted of five steps (Figure 1). Initially, two separate translations of the original English module into a Finnish version were created by Finnish native speakers fluent in English and knowledgeable about the subject in question. Based on these two translations, a final coherent version was agreed upon. This coherent version was then translated back into English by a professional bilingual translator. The translated version was compared with the original module by the license holder and, based on the feedback, the Finnish version was created. Final amendments to the questionnaire were made following the pilot study comments.
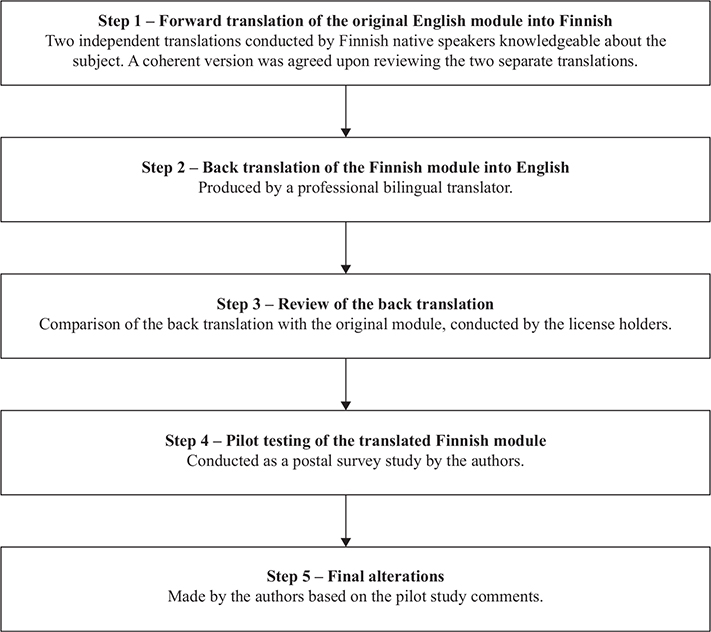
Figure 1. Flow chart showing the translation process behind the Finnish FACE-Q Head and Neck Cancer Module.
Pilot study
Finnish-speaking patients who underwent resection and reconstruction of the oral cavity, tonsil, or tongue area at the Helsinki University Hospital between 2019 and 2021 were identified using theatre logs. Alive patients without advanced conditions affecting memory or cognition were included in the pilot study, conducted as a postal survey. The participants were asked to fill the newly translated Finnish version of the module. In addition, a section for commenting on any linguistic ambiguities, inconsistencies, or comprehensibility issues was added on each page of the module, enabling participants to comment on any language issues they identified while filling out the questionnaire. Furthermore, patients were allowed to suggest alternative phrasings and wordings, for expressions they found confusing or clumsy. Information on the study and a written consent form was included. A prepaid envelope for returning the completed module was provided. Patients who did not respond within 2 weeks were approached a second time.
Data processing
Data from the comments and suggestions regarding the language of the module and the answers to the FACE-Q Head and Neck Cancer Module scales reported by the patients were collected. The results from the pilot study were analysed according to the guidelines established by the module developer [13]. The scores for each of the 14 scales were collected separately. Scales with more than 50% of items unanswered were completely excluded from the analysis. Scales with fewer than 50% of the answers missing were supplemented with the average score of the completed sections of that same scale [13]. The total score for each scale was converted to a range from 0 to 100, using the published Rash conversion [13]. All data were analysed using SPSS (version 28.0.0) [22].
Ethics
Approval for the study protocol was granted by the ethics committee of Helsinki University Hospital, and the research permit was obtained from the division of Musculoskeletal and Plastic Surgery, Helsinki University Hospital. All participants provided written, informed consent.
Results
Linguistic translation of the module
The FACE-Q Head and Neck Cancer Module translated readily into Finnish. No major issues with either vocabulary or phrasing of the module were discovered throughout the five-step translation process. Based on the comparison between the original English and the back-translated questionnaire, no alterations were made to the Finnish version of the module.
Validation of the translation
Based on the feedback from the pilot study participants regarding the language of the module, two changes were made to the wording of the module (Table 1). The word ‘self-conscious’ (‘Itsetietoinen’ in Finnish), which was used in four of the 14 translated scales, was reported by several participants to be confusing and leaving room for interpretation. Based on this response, the word was changed to a synonym, ‘vaivaantunut’,which has a more negative connotation than the original translation. This term was changed in all four scales to clarify the intention behind the question. In addition, one question was rephrased based on a suggestion from one participant, making the question easier to understand. Otherwise, no further linguistic deficiencies were reported by the pilot study participants, in either wording or phrasing for instructions, questions, or answer options in the newly translated FACE-Q Head and Neck Cancer Module.
Pilot study
A total of 51 patients filling the inclusion criteria were identified and approached. Twenty-one (41%) of the approached patients returned the filled-out survey package with the newly translated FACE-Q Head and Neck Cancer Module. The median age of the participants at the time of the pilot study was 66 years, with a range of 48–89 years, and the median time since reconstructive surgery was 3 years, ranging from 1 to 4 years (Table 2).
The median scores obtained from this pilot study, along with the cohort mean scores from the original validation study are presented in Table 3 [13]. In all items, except the ‘Cancer Worry’ item, a higher score indicated a better outcome. A higher score in the ‘Cancer Worry’ item suggested more distress, which was considered a worse outcome.
| Pilot study | Original validation study | ||
| Median score | Score range (minimum-maximum) | Cohort mean score [13] | |
| Function scales | |||
| Appearance | 89 | 50–100 | 64.7 |
| Eating and drinking | 57 | 29–100 | 52.4 |
| Oral competence | 75 | 41–100 | 55.7 |
| Salivation | 62 | 33–100 | 46.9 |
| Smiling | 88 | 34–100 | 58.8 |
| Speaking | 50 | 0–100 | 47.7 |
| Swallowing | 73 | 0–100 | 53.1 |
| Distress scales | |||
| Appearance distress | 75 | 41–100 | 64.74 |
| Drooling distress | 100 | 0–100 | 73.2 |
| Eating distress | 50 | 0–100 | 61.4 |
| Smiling distress | 78 | 31–100 | 71.2 |
| Speaking distress | 63 | 25–100 | 62.8 |
| Cancer worry | 29 | 0–55 | 31.5 |
| Information | 89 | 35–100 | Not available |
Discussion
The importance of understanding the impact of surgical treatments on the patient’s health-related quality of life is increasingly valued [23, 24]. For patients with HNC, the potential effects the disease and its treatment may have on their functionality, appearance and psychosocial well-being are considerable and should not be underestimated. Understanding this impact is crucial for guiding supportive interventions and potentially improving the existing treatment options. In this study, a linguistically validated Finnish version of the FACE-Q Head and Neck Cancer Module was produced, a PROM specifically designed for the evaluation of the health-related quality of life of HNC patients [13].
Minimal adjustments to the phrasing and wording of the module were needed throughout the translation process of the original module into the first version of the Finnish module. This easy adaptation of the questionnaire into Finnish illustrates the benefits of the forward translation being performed by medical professionals familiar with the subject matter, fluent in the original language and native speakers of the target language. Awareness of the target population, in particular the difference between medical terminology and common language, is also key [25]. In line with this, the recruited patients broadly represented the target population, in terms of both age and gender, with nearly equal representation of men and women. The input gathered from the participants, invited to provide feedback on the module language, led to the identification of two language-related issues in the phrasing of the module that were not detected during the translation process, emphasising the importance of conducting a pilot study during the translation process.
The general scores for the domains assessing facial functionality, facial appearance and psychosocial distress were consistently in the upper end of the 0 to 100 scale. The results observed in our pilot study population were higher for most of the items within these domains, compared to the results obtained in the original validation study conducted with 219 US patients [13]. However, the small size of our study group prevented us from conducting any statistical comparison. This trend was noticeable in scales evaluating facial appearance, smiling, oral competence and swallowing [13]. In particular, the pilot study participants reported less distress related to smiling, appearance and drooling [13]. On the contrary, the pilot study participants expressed more distress regarding eating than the participants in the original validation study [13]. The apparent differences between the two studies are most likely caused by differences in population size and patient characteristics, rather than deficiencies in the translation. Our pilot study included a small population, with most patients having cancer located in various parts of the tongue. The impact of surgery on facial appearance and functionality in this area may be comparatively lower than in regions such as the jaw, a patient group, which was more extensively represented in the original validation study [13]. In line with this, scales assessing functionality more dependent on the tongue, for example, eating, speaking, and drinking, displayed results more aligned with each other. No notable differences in cancer worry or speaking distress were observed between the pilot study and the validation study participants [13].
The number of participants in our study group, although insufficient for statistical analyses or results comparison, can still be considered sufficient for the purpose of this study [26]. In addition, the pilot study patients had undergone resections and reconstructions of variable extent and localisation, reducing the generalisability of our results. However, this patient diversity can also be considered a strength of our study, as it allowed for the testing of the module’s comprehensibility and relevance across a broad spectrum of patients in terms of both age and gender, representing the typical patient population at our institution. Another limitation of our study is the use of a postal survey format. However, to mitigate this, a separate section on each page of the module was included, following each scale, for patients to comment on linguistic challenges and suggest alternative phrasings. Although conducting interviews with the pilot study participants might have brought additional insights into the module language, the fact that most participants identified the same deficiencies in items within the same scales, suggests that this method was effective at highlighting the most significant issues in the translation. These issues were then addressed before the final formatting of the Finnish FACE-Q Head and Neck Module.
In conclusion, the newly translated and linguistically validated Finnish version of the FACE-Q Head and Neck Cancer Module translated well into Finnish and showed results that were in line with the results obtained from the original validation study [13]. Further research is needed to better evaluate the performance and behaviour of the module within the Finnish population. The Finnish FACE-Q Head and Neck Cancer Module is available free of charge at the Q-Portfolio Website [17].
Acknowledgements
The authors would like to thank the representatives at the Q-portfolio for their help with the project.
Authors’ contributions
All authors contributed to the conception and design of the study. LV, AL, PL and PH conducted the translation process of the Finnish FACE-Q Head and Neck Cancer Module. LV, IBH and AH collected and analyzed the pilot study data. LV drafted the first version of the manuscript. All authors contributed to and approved the final version of the manuscript.
ORCID
Lotta Varakas, MB,  https://orcid.org/0009-0003-9732-246X
https://orcid.org/0009-0003-9732-246X
Ian Barner-Rasmussen, PhD,  https://orcid.org/0000-0001-6294-4988
https://orcid.org/0000-0001-6294-4988
Aaro Haapaniemi, PhD,  https://orcid.org/0000-0003-3097-1118
https://orcid.org/0000-0003-3097-1118
Andrew Lindford, PhD,  https://orcid.org/0000-0002-1120-8160
https://orcid.org/0000-0002-1120-8160
Patrik Lassus, PhD,  https://orcid.org/0000-0003-0554-5096
https://orcid.org/0000-0003-0554-5096
Pauliina Homsy, PhD,  https://orcid.org/0000-0002-5454-3313
https://orcid.org/0000-0002-5454-3313
References
[1] Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. https://doi.org/10.1038/s41572-020-00224-3
[2] Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24(3):379–396. https://doi.org/10.1016/j.soc.2015.03.001
[3] Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dental J. 2022;233(9):780–786. https://doi.org/10.1038/s41415-022-5166-x
[4] Guo K, Xiao W, Chen X, Zhao Z, Lin Y, Chen G. Epidemiological trends of head and neck cancer: a population-based study. Biomed Res Int. 2021;2021:1738932. https://doi.org/10.1155/2021/9758328
[5] Alfouzan AF. Radiation therapy in head and neck cancer. Saudi Med J. 2021;42(3):247–254. https://doi.org/10.15537/smj.2021.42.3.20210660
[6] Albornoz CR, Pusic AL, Reavey P, Scott AM, Klassen AF, Cano SJ, et al. Measuring health-related quality of life outcomes in head and neck reconstruction. Clin Plast Surg. 2013;40(2):341–349.
[7] Ringash J, Bernstein LJ, Devins G, Dunphy C, Giuliani M, Martino R, et al. Head and neck cancer survivorship: learning the needs, meeting the needs. Semin Radiat Oncol. 2018;28(1):64–74. https://doi.org/10.1016/j.semradonc.2017.08.008
[8] Loewen I, Jeffery CC, Rieger J, Constantinescu G. Prehabilitation in head and neck cancer patients: a literature review. J Otolaryngol Head Neck Surg. 2021;50(1):2. https://doi.org/10.1186/s40463-020-00486-7
[9] El-Deiry M, Funk GF, Nalwa S, Karnell LH, Smith RB, Buatti JM, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131(10):879–885. https://doi.org/10.1001/archotol.131.10.879
[10] Zebolsky AL, Patel N, Heaton CM, Park AM, Seth R, Knott PD. Patient-reported aesthetic and psychosocial outcomes after microvascular reconstruction for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(12):1035–1044. https://doi.org/10.1001/jamaoto.2021.1563
[11] Djan R, Penington A. A systematic review of questionnaires to measure the impact of appearance on quality of life for head and neck cancer patients. J Plast Reconstr Aesthet Surg. 2013;66(5):647–659. https://doi.org/10.1016/j.bjps.2013.01.007
[12] Zebolsky AL, Ochoa E, Badran KW, Heaton C, Park A, Seth R, et al. Appearance-related distress and social functioning after head and neck microvascular reconstruction. Laryngoscope. 2021;131(7):E2204–E2211. https://doi.org/10.1002/lary.29548
[13] Cracchiolo JR, Klassen AF, Young-Afat DA, Albornoz CR, Cano SJ, Patel SG, et al. Leveraging patient-reported outcomes data to inform oncology clinical decision making: introducing the FACE-Q Head and Neck Cancer Module. Cancer. 2019;125(6):863–872. https://doi.org/10.1002/cncr.31900
[14] Cohen WA, Albornoz CR, Cordeiro PG, Cracchiolo J, Encarnacion E, Lee M, et al. Health-related quality of life following reconstruction for common head and neck surgical defects. Plast Reconstr Surg. 2016;138(6):1312–1320. https://doi.org/10.1097/PRS.0000000000002766
[15] Pusic A, Liu JC, Chen CM, Cano S, Davidge K, Klassen A, et al. A systematic review of patient-reported outcome measures in head and neck cancer surgery. Otolaryngol Head Neck Surg. 2007;136(4):525–535. https://doi.org/10.1016/j.otohns.2006.12.006
[16] Ellis MA, Sterba KR, Brennan EA, Maurer S, Hill EG, Day TA, et al. A systematic review of patient-reported outcome measures assessing body image disturbance in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(6):941–954. https://doi.org/10.1177/0194599819829018
[17] FACE-Q Head & neck cancer – user’s guide. [cited 2024 7 th Jan]. Available from: https://qportfolio.org/face-q/head-neck-cancer/
[18] Pagotto VPF, Lobato RC, Bustillo AMB, Lopes CP, Tutihashi RMC, Busnardo FDF, et al. FACE-Q head and neck cancer questionnaire for Brazilian Portuguese: translation, cross-cultural adaptation, and linguistic validation. Revista Brasileira De Cirurgia Plástica. 2022;37(3): 302-307 https://doi.org/10.5935/2177-1235.2022RBCP.591-en
[19] de Jel Day-A DVC, Rakhorst HA, Dirven R, Smeele LE. P-148 FACE-Q head and neck cancer module: first results of a Dutch multicenter cohort cross-sectional validation study. Oral Oncol. 2021;118:12. https://doi.org/10.1016/S1368-8375(21)00435-8
[20] Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for Patient-Reported Outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. https://doi.org/10.1111/j.1524-4733.2005.04054.x
[21] FACE-Q – head and neck cancer module. 2024. Available from: https://eprovide.mapi-trust.org/instruments/face-q-head-and-neck-cancer-module
[22] IBM SPSS Statistics 28.0.0. [cited 7th Jan 2024]. Available from: https://www.ibm.com/spss
[23] Sharma K, Steele K, Birks M, Jones G, Miller G. Patient-reported outcome measures in plastic surgery: an introduction and review of clinical applications. Ann Plast Surg. 2019;83(3):247–252. https://doi.org/10.1097/SAP.0000000000001894
[24] Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. https://doi.org/10.1001/jama.2017.7156
[25] Krogsgaard MR, Brodersen J, Christensen KB, Siersma V, Jensen J, Hansen CF, et al. How to translate and locally adapt a PROM. Assessment of cross-cultural differential item functioning. Scand J Med Sci Sports. 2021;31(5):999–1008. https://doi.org/10.1111/sms.13854
[26] Mokkink LB, Prinsen C, Patrick DL, Alonso J, Bouter LM, De Vet H, et al. COSMIN Study Design checklist for Patient-reported outcome measurement instruments. COSMIN: Amsterdam, The Netherlands; 2019. p. 1–32.