ORIGINAL RESEARCH ARTICLE
Spring-assisted posterior vault expansion in children over 2 years of age with craniosynostosis
Karin Säljö, MD, PhDa,b, Madiha Bhatti-Søfteland, MD, PhDa,b, Peter Tarnow, MD, PhDa,b, Robert Olsson, MDc, Tobias Hallén, MD, PhDc, Wen-Chih Chao, BScd, Lars Kölby, MD, PhDa,b and Giovanni Maltese, MD, PhDa,b
aDepartment of Plastic Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bRegion Västra Götaland, Sahlgrenska University Hospital, Department of Plastic Surgery, Gothenburg, Sweden; cDepartment of Neurosurgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; dImaging Lab, Department of Radiology, Sahlgrenska University Hospital, Gothenburg, Sweden
ABSTRACT
Background: This study evaluated spring-assisted posterior vault expansion (SA-PVE) in children aged > 2 years with craniosynostosis and signs of high intracranial pressure (ICP).
Methods: We retrospectively analysed all consecutive patients aged > 2 years and operated with SA-PVE between 2018 and 2020 at the Craniofacial Center at Sahlgrenska University Hospital, Sweden. During the procedure, a circumferent occipital bone flap extending below the torcula was created and remained attached to the dura. Intracranial volumes (ICVs) were calculated from computed tomography (CT) images, and demographic data and information regarding symptoms and signs of high ICP were collected.
Results: The study included eight patients [Crouzon/Pfeiffer (n = 4), multiple craniosynostosis (n = 3), and secondary synostosis (n = 1)]. Median age at SA-PVE was 3.8 years (range: 2.5–12.8 years), and springs were removed after a median of 5.5 months (range: 2.3–8.3 months). The median operating time was 164 min (range: 102–221 min), and estimated blood loss was 4.5 mL/kg body weight (range: 1.4–59.1 mL/kg body weight), with 50% of patients receiving a blood transfusion. The median increase in ICV was 206 cm3 (range: 122–344 cm3) representing an 18.7% increase (range: 7.9–24.1%; p = 0.01). We observed no major perioperative complications, and symptoms related to high ICP were improved or absent at clinical follow-up.
Conclusion: These results demonstrated that SA-PVE involving creation of a large occipital bone flap including the torcula as a safe and effective surgical treatment in children aged >2 years with craniosynostosis and elevated ICP.
KEYWORDS: Craniosynostosis; syndromic craniosynostosis; spring-assisted posterior vault expansion; high intracranial pressure; intracranial volume
Citation: Journal of Plastic Surgery and Hand Surgery 2024; 59: 117–112. DOI: https://doi.org/10.2340/jphs.v59.41906.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 4 June 2024; Accepted: 27 August 2024; Published: 25 September 2024
CONTACT Karin Säljö, MD, PhD karin.saljo@vgregion.se Department of Plastic Surgery, Sahlgrenska University Hospital, University of Gothenburg Institute for Clinical Sciences, Gothenburg SE-413 45, Sweden
Supplemental data for this article can be accessed online at https://doi.org/10.2340/jphs.v59.41906
Competing interests and funding: This study was supported by grants from the Swedish State under the agreement between the government and the county councils, the ALF-agreement (ALFGBG-716621, ALFGBG-971618), and Frimurare Barnhusdirektionen in Gothenburg. The authors declare no conflicts of interest.
Introduction
Complex craniosynostosis is a congenital malformation with multiple prematurely fused cranial sutures and often associated with syndromes, such as Apert and Crouzon. Children with multi-suture craniosynostosis are at high risk of developing increased intracranial pressure (ICP) and consequent sequelae, such as optic nerve atrophy and neurocognitive deficits. Surgical techniques to expand the cranial vault and consequently increase intracranial volume (ICV) aim to improve head shape and reverse ICP. Posterior skull expansion with circumferential occipital craniectomy was first described by Sgouros et al. [1]. During this procedure, removal of a 2-cm-wide strip of occipital bone creates a free-floating bone flap. Modifications to the procedure include the size and area of the posterior skull included in the bone flap, as well as the methods used for fixation. Recent methodological evolutions include the use of dynamic techniques assisted by distractors or springs [2, 3]. Di Rocco et al. [4] demonstrated posterior cranial vault expansion with internal distractors using distraction osteogenesis as an efficient surgical treatment for patients up to 15 years of age with craniosynostosis and elevated ICP. Other reports also suggest spring-assisted posterior vault expansion (SA-PVE) as an effective technique for surgical correction of multi-suture craniosynostosis during the first years of life [5–8]. In these studies, the caudal osteotomy lines are placed above the torcula and the posterior bone flap is partially attached to the occipital bone or statically fixed with wires. These strategies aim to avoid the torcula area (i.e. the confluence of sinuses) and detachment of the bone flap.
In the present study, we describe a version of the SA-PVE technique developed at the craniofacial unit at Sahlgrenska University Hospital, Gothenburg, Sweden. This approach encompasses the torcula in the bone flap to facilitate expansion in the posterior/occipital skull, thereby enhancing subsequent increases in ICV. Furthermore, we present the results of this form of SA-PVE in children aged > 2 years with complex craniosynostosis and signs of high ICP.
Materials and methods
Patient selection and data
We retrospectively analysed all consecutive patients aged > 2 years of age and operated with SA-PVE between 2018 and 2020 at the Craniofacial Unit at Sahlgrenska University Hospital. Demographic data and perioperative outcomes, including operation time, estimated blood loss, blood transfusion volumes and length of stay (LOS), were collected. Blood loss as a percentage of total blood volume was calculated assuming a total blood volume of 75 mL/kg body weight for children aged between 2 and 8 years and 70 mL/kg body weight for those aged > 9 years of age. The presence of symptoms and objective signs of high ICP, such as headache, nausea and ophthalmoscopic findings (e.g. papilledema), were evaluated before and after surgery and up to 1-year post-surgery.
Surgical technique
Patients were placed in a prone position, and a circumferent occipital osteotomy was performed, with the caudal osteotomy extending below the torcula (Figure 1). The occipital bone flap remained attached to the dura, and six manually manufactured 8N springs were placed along the osteotomy.
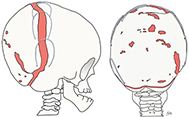
Figure 1. Illustrated description of the surgical procedure, including osteotomies and positioning of the springs.
Radiologic evaluation and intracranial volume measurement
Low-dose computed tomography (CT) was performed prior to the operation, at the time of spring removal, and at 1-year post-surgery. Intracranial volumes were calculated from the CT images using Syngo. Via 30 software (Siemens, Berlin, Germany) at the Imaging and Intervention Centre at Sahlgrenska University Hospital. The upper and lower boundaries of the volume determinations were established at the inside of the vertex to the foramen magnum according to the point at which the dens appeared in the coronal section (Figure 2).
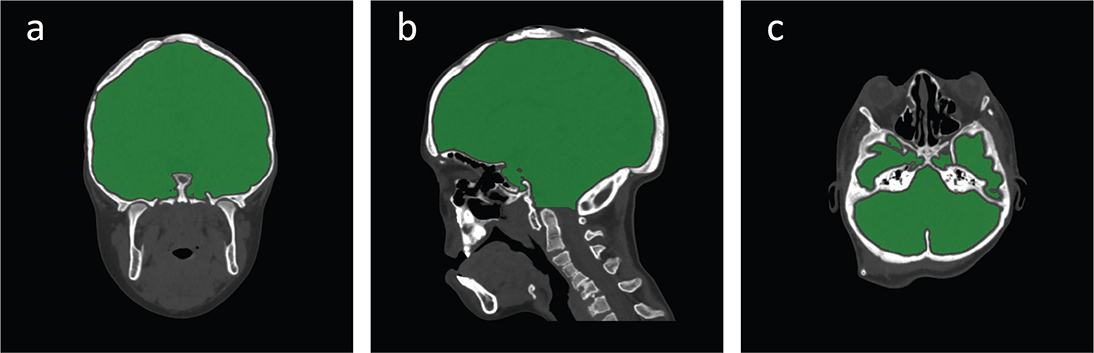
Figure 2. Illustrated description of the axial (a), sagittal (b), and coronal (c) limitations.
Statistical analysis
Patient-specific data, as well as peri- and post-operative outcomes, are presented as medians, interquartile ranges (IQRs), and ranges because of the absence of a normal distribution in the data. All volume measurements were performed in triplicate by a single observer, with the mean value presented for each patient and median values with an IQR and range presented for the cohort. Cronbach’s alpha was calculated to evaluate the intra-rater reliability of the volume measurements. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) (v29.0; IBM Corp., Armonk, NY, USA). Pre- and post-operative volumes were compared using the Wilcoxon signed rank test. For sensitivity analysis, we employed a mixed-effect model with individual random effects to estimate the effect of treatment. This allowed use of all original measurements (six measurements for each patient) and accounted for fluctuations between different measurements for the same individual. All p-values were two-sided, and a p < 0.05 was considered statistically significant.
Ethical considerations
The study was approved by the Gothenburg Ethics Committee (approval No. 748:11).
Results
Patient population and characteristics
We identified a total of 15 patients aged > 2 years and operated with-SA-PVE. Four patients lacked adequate CT imaging prior to surgery and three of the identified patients underwent concomitant fronto-orbital advancement (FOA) during the procedure and were thus excluded. The remaining eight patients [Crouzon/Pfeiffer (n = 4), multiple/complex craniosynostosis (n = 3), and secondary synostosis because of shunt over-drainage in a patient with benign tumour in corpus pineal and aqueductal stenosis (n = 1)] were included in the analysis (Table 1). Indications for surgery were diagnosis with high ICP using invasive measurements (n = 4), presence of papilledema (n = 3) and/or Chiari malformation (n = 3). Five of the included patients presented with severe headache and four with hydrocephalus, of which two also required ventriculoperitoneal shunt treatment. Seven of the patients had previously undergone cranioplasty, with five of those procedures including FOA.
Perioperative and surgical outcomes
We observed no major perioperative complications, such as bleeding or dural tears, according to Leeds classification [9]. Tables 1 and 2 summarise the perioperative outcomes. The median age at SA-PVE was 3.8 years (IQR: 6.7 years; range: 2.5–12.8 years). Median body weight at surgery was 15.5 kg (IQR: 20.9 kg; range: 11–42 kg) and median operation time was 164 min (IQR: 81 min; range: 102–221 min). The median estimated blood loss was equivalent to 4.5 mL/kg body weight (IQR: 13.0 mL/kg body weight; range: 1.4–59.1 mL/kg body weight) representing 6.5% (IQR: 18.5%; range: 1.9–78.8%) of the estimated blood volume of each child. Intraoperative transfusion volume was 78.5 mL (IQR: 434 mL; range: 0–520 mL) equivalent to 1.9 mL/kg body weight (IQR: 16.2 mL/kg body weight; range: 0–47.3 mL/kg body weight). Four patients (50%) did not require a blood transfusion during the peri- or post-operative period, and one patient received a blood transfusion (100 mL) during the post-operative period. Median hospital LOS after SA-PVE was 5 days (IQR: 4.3 days; range: 3–9 days).
The median springs removal was 5.5 months (IQR: 3.4 months; range: 2.3–8.3 months) after insertion. In two patients, the springs were removed early because of exposure or risk from exposure of the springs, and one patient had a superficial wound over one spring after receiving minor head trauma. We observed no clinically relevant post-operative infections. The median LOS after spring removal was 1 day (IQR: 1 day; range: 0–12 days). In one patient, spring removal was performed during a hospital stay because of shunt-related problems that resulted in an LOS of 12 days.
The median pre- and post-operative ICVs were 1,215 cm3 (IQR: 421 cm3; 910–1,535 cm3) and 1,453 cm3 (IQR: 438 cm3; 1,097–1,772 cm3), respectively. The increase in ICV was 206 cm3 (IQR: 76 cm3; range: 122–344 cm3) representing a volume increase of 18.7% (IQR: 7.7%; range: 7.9–24.1%) from spring insertion to removal (p = 0.01) (Table 3; Figure 3; and Figure, Supplemental Digital Content 1). Sensitivity analysis estimated the treatment effect at 215.875 [standard error (SE) = 12.886; p < 0.001], which remained consistent following the addition of age as a control variable. The effect remained evident at 1-year post-surgery.
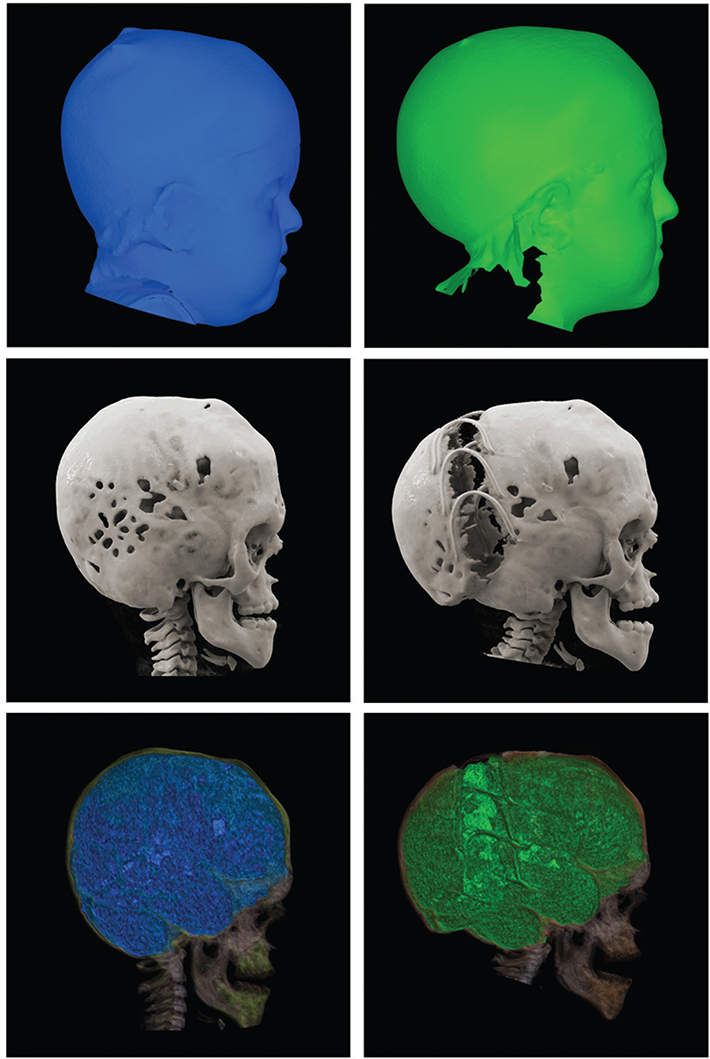
Figure 3. Illustration with 3D photos (a, b) and reconstructions of CT scans (c, d) and volume measurements (e, f) before (a, c, e) and after (b, d, f) surgery in a patient with multiple craniosynostoses operated with SA-PVE at 4.3 years of age. Post-surgery ICV increased by 272 cm3 representing a 22.9% expansion. CT: computed tomography; SA-PVE: spring-assisted posterior vault expansion; ICV: intracranial volume.
Analyses of pre- and post-operative ICV measurements resulted in an overall Cronbach’s alpha of 0.995. Hence, excellent consistency and high intra-rater reliability of the ICV measurements. We also tested the interaction between treatment effect and age (−6.220; SE = 3.407; p = 0.076). Although the results suggested that the difference in ICV between pre- and post-surgery depended on age, the small sample size of the cohort was insufficient to allow a rigorous determination of such an association (Figure, Supplemental Digital Content 2).
Symptoms related to high ICP, such as headache, nausea and irritability, improved significantly or were absent during clinical follow-up at 4-weeks and 1-year post-surgery. Additionally, the papilledemas present before the operation (n = 4) were normalised post-surgery. Computed topography and magnetic resonance imaging (MRI) scans at 1-year post-surgery showed good bone healing across the osteotomies and either regression of or no changes to the Chiari malformation. None of the three patients presenting Chiari malformation required foramen magnum decompression following surgery nor have they undergone FOA/anterior expansion during the 3–5-year follow-up period.
Discussion
Dynamic posterior vault expansion (PVE) via distraction osteogenesis or spinal spinal anesthesia (SA) surgery has become a commonly used technique in craniofacial centres based on its ability to achieve greater volume expansion relative static techniques [10]. Dynamic expansion of the posterior vault requires less-extensive dural dissection, decreases subsequent risk for perioperative bleeding requiring transfusion, and is, therefore, considered a much less invasive surgical method. Its use as a first-stage procedure in infants with complex craniosynostosis to prevent elevated ICP is currently gaining popularity.
Children with complex craniosynostosis are at high risk of developing elevated ICP and consequently suffering from related symptoms. The present study shows that SA-PVE can be safely performed later in life to treat fortified, fully developed, elevated ICP. Our study cohort included a consecutive series of eight children aged >2 years (up to 13 years) with complex craniosynostosis and radiographic and/or clinical signs of elevated ICP. The surgical strategy was SA-PVE, which created a bone flap encompassing the torcula and remaining attached to the dura. The median increase in ICV following this procedure was 18.7% (206 mL) in the absence of major bleeding and requiring low total perioperative blood transfusion volume (1.9 mL/kg body weight). Furthermore, only 50% of the patients that underwent this procedure required blood transfusion during the perioperative period, and all patients showed considerable symptomatic and radiographic improvements post-surgery.
Conventional static expansion of the posterior vault requires extensive dural dissection and presents a subsequent risk of blood loss. In these procedures, the skin envelope also restrains the underlying PVE. By contrast, dynamic expansion is less damaging to soft tissue and conceivably allows more expansion. Different dynamic strategies for PVE using internal distractors or springs have been developed and are frequently used in children with craniosynostosis [2, 4, 11, 12]. However, there remains no consensus on the correct timing, surgical sequence or technical aspects of these procedures.
An evaluation by Di Rocco et al. [4] of outcomes of 12 children (median age: 8.6 months; range: 3 months to 15 years) with craniosynostosis treated with PVE using internal distractors revealed a median increase in ICV of 13.9% (132 mL) at 117 days post-surgery. In the present study, we identified an increased ICV of 18.7% (206 mL) in a significantly older population (mean age: 3.8 years; range: 2.5–12.8 years) in which the calvarial growth is less. In the first review of SA-PVE outcomes relative to those of conventional techniques in children with craniosynostosis aged < 2 years, Jong et al. [5] found larger increases in skull circumference and anterior–posterior length, as well as decreased blood loss, in those undergoing SA-PVE. Moreover, a recent study from Great Ormond Street Hospital (GOSH) identified SA-PVE as an effective technique for increasing ICV and resolving elevated ICP in a cohort of children from their craniofacial unit (mean age: 2.3 years; range: 3 months to 5.6 years) [7]. They also reported a significant increase in ICV of 248 ± 172 cm3 (27 ± 22%) following SA-PVE, which is consistent with our findings (206 cm3, IQR: 76 cm3; 18.7%, IQR: 7.7%). However, that study performed a slightly different surgical technique in a younger cohort relative to the present study. Furthermore, they concluded that younger patients with a markedly brachycephalic head shape showed higher volumetric increases, suggesting that this cohort might benefit from additional surgery. Notably, the present study found that age-related trends were negatively correlated with volume increases; although, the small sample size did not allow for proper testing of this association.
Although there is substantial risk of bleeding and a need for transfusion during PVE, the methods used to estimate blood loss and required transfusion volumes are inexact. In a cohort of > 1,000 patients that underwent cranial vault remodelling for craniosynostosis, Chow et al. [13] identified that perioperative blood transfusion of > 60 mL/kg body weight was associated with increased risk for complications and prolonged hospital LOS. Additionally, this threshold was identified as an independent predictor of complications, whereas transfusion volumes of 25 mL/kg body weight had no significant effect on postoperative outcomes. The Leeds classification of complications in craniosynostosis surgery applies a threshold for excessive blood transfusion at > 60 mL/kg body weight [9]. In the present study, we reported a total perioperative blood transfusion volume of 1.9 mL/kg body weight and identified no major bleeding according to the Leeds classification.
In a comparison of children with syndromic craniosynostosis treated with expansion of the posterior cranial vault using distraction osteogenesis or conventional posterior vault remodelling, Steinbacher et al. [11] reported significantly less blood loss in the group undergoing distraction. However, the mean estimated blood loss after dynamic expansion was 487 mL (range: 300–2,000 mL), corresponding to 52% of total blood volume, and seven of eight patients received perioperative blood transfusion. In the present study, we reported a median estimated blood loss of 125 mL (range: 20–650 mL) representing 6.5% of total blood volume, and only four of eight children required perioperative blood transfusion (median volume: 78.4 mL). Our findings are in line with those of a large retrospective study of 200 patients (mean age: 19 months; range: 2–131 months) that underwent SA-PVE, in which 122 patients received a median blood transfusion volume of 196 mL [8]. In the present study, only one patient presented a high estimated volume of bleeding (59.0 mL/kg body weight; 78.8% of total blood volume) and consequently received a blood transfusion of 56.4 mL/kg body weight during the perioperative period.
Among the advantages of SA-PVE relative to posterior expansion, the latter involves semi-buried distractor devices. In SA-PVE, the springs are fully buried and less prone to infections or other types of trauma. A recent systematic review reported a wound-infection rate of 27% following posterior cranial vault distraction osteogenesis [12]. In the present study, we identified no clinically relevant wound infections following SA-PVE. Additionally, the absence of transcutaneous pins encourages a better quality of life for school-aged patients and does not require parental compliance for daily activation of the device. Furthermore, the operation time was considerably longer in the cohort undergoing expansion with distractors (mean: 3.8 h; range: 2.6–5.0 h) [11] relative to that of the cohort in the present study undergoing SA-PVE (mean: 2.7 h; range: 1.7–3.7 h) and the large cohort from the GOSH study (mean: 2.5 h; range: 1.0–5.5 h) [8].
In some studies reporting SA-PVE outcomes, the osteotomies are incomplete and left attached to the bone and dura as a 1-cm strip [5] or the occipital part of the flap remains interconnected to the posterior skull [7,8]. Misier et al. [7] reported six patients with a fully excised osteotomy above the torcula, with the flap fixed to the surrounding skull with wires. This differs from the surgical strategies used in the present study, which entailed a completely released occipital bone flap that remained attached to the dura (Figure 1) in order to retain dura-periosteal/bone circulation.
In the study cohort, we observed no evidence of a collapsed bone flap or development of a large occipital ridge during clinical follow-up. However, three patients had titanium plates or resorbable plates inserted across the osteotomies during spring removal in order to stabilise the remaining ossification defects. Additionally, some studies using SA-PVE report positioning the caudal horizontal osteotomies above the torcula [5, 7]. In the present study, we found that performing osteotomies below the torcula resulted in an estimated blood loss of 4.5 mL/kg body weight (6.6% of total blood volume) and a blood transfusion volume of only 1.9 mL/kg body weight in 50% of the children. These findings are relative to those reported in previous studies using dynamic procedures and despite the occipital bone flap encompassing the torcula.
Conclusions
This study is the first describing SA-PVE using a technique encompassing the torcula in a cohort of consecutively operated children and adolescents. The results of surgical intervention included a volume expansion of 18.7%, no indications of local or systemic complications or infections and low levels of perioperative blood loss. The patient cohort demonstrated substantial improvements in objective and symptomatic signs of high ICP along with sustained improvements observed during clinical and radiological follow-up at 1-year post-surgery. Furthermore, none of the patients with Chiari malformation needed foramen magnum decompression following surgery, and none of the patients has undergone FOA/anterior expansion during the follow-up period (3–5 years). Our findings suggest that SA-PVE with circumferential osteotomy is a safe and effective surgical intervention to treat craniosynostosis and elevated ICP in children up 13 years of age.
Acknowledgments
The authors thank Jennifer Kuhn and Anna Paganini for their assistance with data collection, Niclas Löfgren for excellent imaging processing and illustrations, and Jason Fye, PhD, MS, for English language editing.
ORCID
Karin Säljö  http://orcid.org/0000-0002-2839-9362
http://orcid.org/0000-0002-2839-9362
References
[1] Sgouros S, Goldin J, Hockley A, et al. Posterior skull surgery in craniosynostosis. Childs Nerv Syst. 1996;12:727–733. https://doi.org/10.1007/BF00366158
[2] White N, Evans M, Dover MS, et al. Posterior calvarial vault expansion using distraction osteogenesis. Childs Nerv Syst. 2009;25:231–236. https://doi.org/10.1007/s00381-008-0758-6
[3] Lauritzen CGK, Davis C, Ivarsson A, et al. The evolving role of springs in craniofacial surgery: the first 100 clinical cases. Plast Reconstr Surg. 2008;121:545–554. https://doi.org/10.1097/01.prs.0000297638.76602.de
[4] Di Rocco F, Usami K, Protzenko T, et al. Results and limits of posterior cranial vault expansion by osteotomy and internal distractors. Surg Neurol Int. 2018;9:217. https://doi.org/10.4103/sni.sni_465_17
[5] de Jong T, van Veelen ML, Mathijssen IM. Spring-assisted posterior vault expansion in multisuture craniosynostosis. Childs Nerv Syst. 2013;29:815–820. https://doi.org/10.1007/s00381-013-2033-8
[6] Tunçbilek G, Kaykçoğlu A, Bozkurt G, et al. Spring-mediated cranioplasty in patients with multiple-suture synostosis and cloverleaf skull deformity. J Craniofac Surg. 2012;23:374–377. https://doi.org/10.1097/SCS.0b013e318240fc4d
[7] Ramdat Misier KRR, Breakey RWF, van de Lande LS, et al. Correlation between head shape and volumetric changes following spring-assisted posterior vault expansion. J Craniomaxillofac Surg. 2022;50:343–352. https://doi.org/10.1016/j.jcms.2021.05.004
[8] Breakey RWF, van de Lande LS, Sidpra J, et al. Spring-assisted posterior vault expansion-a single-centre experience of 200 cases. Childs Nerv Syst. 2021;37:3189–3197. https://doi.org/10.1007/s00381-021-05330-5
[9] Shastin D, Peacock S, Guruswamy V, et al. A proposal for a new classification of complications in craniosynostosis surgery. J Neurosurg Pediatr. 2017;19:675–683. https://doi.org/10.3171/2017.1.PEDS16343
[10] Choi M, Flores RL, Havlik RJ. Volumetric analysis of anterior versus posterior cranial vault expansion in patients with syndromic craniosynostosis. J Craniofac Surg. 2012;23:455–458. https://doi.org/10.1097/SCS.0b013e318240ff49
[11] Steinbacher DM, Skirpan J, Puchała J, et al. Expansion of the posterior cranial vault using distraction osteogenesis. Plast Reconstr Surg. 2011;127:792–801. https://doi.org/10.1097/PRS.0b013e318200ab83
[12] Pandey S, Reddy GS, Chug A, et al. Posterior cranial vault distraction osteogenesis: a systematic review. J Oral Biol Craniofac Res. 2022;12:823–832. https://doi.org/10.1016/j.jobcr.2022.09.009
[13] Chow I, Purnell CA, Gosain AK. Assessing the impact of blood loss in cranial vault remodeling: a risk assessment model using the 2012 to 2013 Pediatric National Surgical Quality Improvement Program data sets. Plast Reconstr Surg. 2015;136:1249–1260. https://doi.org/10.1097/PRS.0000000000001783