ORIGINAL RESEARCH ARTICLE
Topical dihydroartemisinin improves wound healing in diabetic mice
Shanshan Shia,b, Yanhong Gongc, Hailiang Hua, Shuai Penga and Ju Liua,b
aSchool of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China; bMedical Research Center, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China; cDepartment of Stomatology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
Impaired skin wound healing is a common complication of diabetes mellitus. Angiogenesis is a critical step in wound healing because it allows oxygen and nutrients to reach the injured area, thereby promoting wound cell proliferation, re-epithelialization, and collagen regeneration. However, the neovascularization of patients with diabetes often decreases. Dihydroartemisinin is an active metabolic product of artemisinin, and it is antimalarial medicine with low toxicity to human beings. This study investigated the effects of topical Dihydroartemisinin on diabetic wound healing. We used a mouse model of the dorsal skin defect in diabetes caused by streptozotocin (STZ) and topically applied Dihydroartemisinin to full-thickness cutaneous wound. Under an advanced positive microscope, the pathological morphology of the wound skin was observed, together with the positive expression of platelet endothelial cell adhesion molecule-1 (CD31) and vascular endothelial growth factor (VEGF). Western blotting and qRT-PCR were used to determine protein and mRNA levels in the wound skin. We found that Dihydroartemisinin improve the healing of wounds in diabetic mice as compared with the untreated control group. In addition, Dihydroartemisinin increases the expression of CD31 and VEGF in diabetic mice. Therefore, Dihydroartemisinin effectively accelerate the process of diabetic wound healing by promoting angiogenesis, implying that Dihydroartemisinin may be used as a topical drug for the treatment of diabetic wounds.
KEYWORDS: Chronic wounds; diabetic wound healing; angiogenesis; dihydroartemisinin
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 26–32. DOI: https://doi.org/10.2340/jphs.v58.5775.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 12 December 2022; Accepted: 3 April 2023. Published: 14 June 2023.
CONTACT Ju Liu ju.liu@sed.edu.cn Medical Research Center, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, 16766 Jingshi Road, Jinan, Shandong 250014, China.
Competing interests and funding: The authors declare that they have no conflicts of interest regarding the publication of this article.
Introduction
Diabetes mellitus result in a number of consequences when persistent hyperglycemia occurs. Epidemiological data suggest that the prevalence of diabetes in adults is increasing annually and is expected to reach 9.9% in 2045 [1]. A serious complication of diabetes is diabetic foot ulcer, which increases patient mortality and disability, and is challenging to cure [2]. Chronic, non-healing lesions are a common sign of diabetic foot ulcers, and they place a significant financial and emotional burden on patients. The development of a non-healing phenotype is tightly linked to inadequate vascular networks, despite the fact that the etiology of chronic, non-healing diabetic wounds varies [3,4]. Insufficient angiogenesis in diabetic wounds results in diminished vascularity and capillary density [5]. Thus, healing of diabetic wounds may be improved by enhanced angiogenesis.
Currently, regulation of angiogenesis and inflammation is the main strategy used to treat diabetic wounds. The use of recombinant human epidermal growth factor hyperbaric oxygen therapy, vascular endothelial progenitor cell therapy, gaseous carbon dioxide transdermal delivery, and other therapeutic methods has been established. Local mast cell therapy, nitric oxide therapy, and negative-pressure trauma therapy can also be used [6,7,8,9,10,11,12]. An emerging treatment is the use of hydrogels [13,14]. Although various techniques have been developed, only a small number of clinical therapies have been widely used to treat diabetic wounds. Consequently, one of the main therapeutic challenges is the development of more efficient treatments for diabetic wounds.
Dihydroartemisinin has demonstrated a potent antiangiogenic effect [15,16] but had a positive effect on angiogenesis in zebrafish [17]. In addition, dihydroartemisinin has been shown to have anti-inflammatory properties [18,19] as well as the ability to modify the immune system and regulate immunological responses [20]. However, the function and mechanism of action of topical dihydroartemisinin in treatment of diabetic wounds have not been investigated. Here, we propose a potential wound-healing hypothesis for topical dihydroartemisinin wounds in a mouse model of diabetes induced by streptozotocin.
Materials and methods
Preparation of dihydroartemisinin
A dihydroartemisinin suspension was prepared by dissolving dihydroartemisinin (125 mg/kg; MedChemExpress, China) in absolute ethanol. This suspension was then stored at 4°C until further use [21,22].
Induction of the diabetic mouse model
Male C57BL/6J were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. We exclusively used male mice to rule out the impact of hormones associated with sexual development on the healing of skin wounds [23]. After a week of adaptation, mice were fasted for 12 h, and streptozotocin was administered via intraperitoneal injection 5 days in a row at a dose of 50 mg/kg/d [24,25,26]. Streptozotocin (Sigma–Aldrich, USA) was freshly dissolved in a pH of 4.5 sterile 0.1 M sodium citrate buffer (Solarbio, China). Blood glucose levels were measured weekly after the streptozotocin injection. Two consecutive blood glucose levels of >300 mg/dL (16.7 mmol/L) were considered diabetic. The wound experiment was performed after 4 weeks and randomly allocated to the indicated groups: nondiabetic, streptozotocin diabetic, and streptozotocin diabetic + dihydroartemisinin treatment. The identical quantity of sodium citrate was administered into the mice of the nondiabetic group.
Dorsal excisional wound model
After 4 weeks of diabetes induction, hair removal was performed on the mice’s backs using a depilator and hair removal cream (Veet, China). Using a sterile 6-mm skin punch biopsy (Acuderm, USA), two full-thickness (skin and panniculus carnosus) excisional incisions were created on the back of each mouse. The wound was covered with a transparent bio-occlusive dressing (Tegaderm; Health Care, USA), thereby creating a moist wound chamber environment [27,28,29,30]. On the second day, mice in the dihydroartemisinin treatment group were treated with 125 mg/kg dihydroartemisinin once a day on the lesion area for 14 days, the nondiabetic group consisted of mice without streptozotocin modeling and after hair removal.
Evaluation of diabetic full-thickness wound healing
Using the ImageJ analysis program, the wound area was determined from the photos taken at days 0, 3, 5, 7, 10, 12, and 14 after wounding and expressed as a percentage of the initial wound area. Wound closure rates were calculated using the following formula: (wound area on day 0 × wound area on day X)/wound on day 0 × 100%.
Hematoxylin-eosin staining and immunohistochemical analysis
The skin of each group was taken with scissors at the specified time point, encompassing the entire wound and the 5 mm-wide surrounding margin of the unwounded skin. The skin samples were then split down the middle of the lesion to acquire the maximum diameter of the wound margin, and they were instantly fixed by dipping them in a 10% formalin solution for histological analysis. The samples were serially sectioned at a thickness of 4 μm, deparaffinized, and rehydrated. Haematoxylin and eosin (H&E) was used to stain the morphology of the wound. Immunohistochemistry was performed using cell adhesion molecule-1 (CD31) antibodies on tissue sections. The sections were de-paraffinised, rehydrated, and heated for antigen recovery in a microwave oven. The sections were incubated overnight at 4°C with anti-CD31 antibody (Abcam, USA). A general two-step kit (ZSGB-BIO, China) was used for incubation, and a DAB kit was used for color development. After counterstaining with hematoxylin, the slides were assessed using an advanced forward fluorescence microscope (Carl Zeiss Microscopy GmbH, Germany).
Qualitative real-time polymerase chain reaction
The expression of vascular endothelial growth factor (VEGF) mRNA was assayed using qRT-RCR. Total RNA was extracted in skin tissues using the TRIzol reagent (Beijing ComWin Biotech Co., Ltd. China), The Fast All-in-One RT Kit (ESscience Biotech) was then used to synthesise cDNA from the extracted total RNA. Real-time polymerase chain reaction (RT-PCR) was performed with 2X Universal SYBR Green Fast qPCR Mix (ABclonal, China). The relative expression levels of each RNA sample were calculated with the standard 2-∆∆Ct method and normalized using the housekeeping gene β-actin. The primer sequences used are listed in Table 1.
Western blotting
VEGF protein expression was detected using Western blotting. Proteins were extracted using Radioimmunoprecipitation assay (RIPA) buffer (Solarbio, China), which contained protease inhibitors. The Bicinchoninic acid assay (Beyotime, China) method was used to quantify the concentration of the collected proteins. The samples were electrophoresed on polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk for 2h. Subsequently, the corresponding anti-VEGFA antibody (1:1000, Abcam, UK) was incubated at 4°C for 12 h. After washing with Tris-buffered saline with Tween solution (TBST), the membranes were incubated with the corresponding secondary antibody (ZSGB-BIO, China) for 1 h at room temperature. Images were visualized using a multicolor fluorescence imaging system (Amersham, UK). Grey level analysis was performed using the ImageJ software.
Statistical analysis
The student’s unpaired 2-talied t test was used for all statistical analyses. Statistical significance was set at p < 0.05.
Results
General conditions of the diabetic mice
C57BL/6J mice were administered streptozotocin by intraperitoneal injection to establish a model of diabetes. All diabetic mice had a blood glucose higher than 16.7 mmol/L seven days after injection, and these levels remained stable throughout the experiment. The weights of all mice were measured at the set time points. The weights of all mice were measured at the set time points. The weight in the nondiabetic group increased rapidly, while that in the streptozotocin diabetic group and the streptozotocin diabetic + dihydroartemisinin treatment group increased slowly and then exhibited poor growth four weeks after injection (Figure 1C).
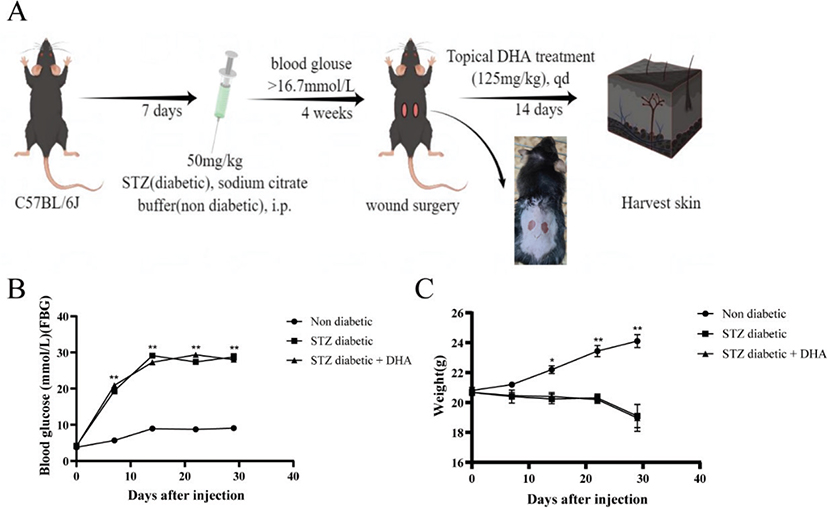
Figure 1. Study design. (A) Schematic representation of the wound healing experiment in nondiabetic and streptozotocin diabetic mice. (B) Changes in blood glucose after injection. (C) Changes in body weight after injection. P values were calculated using the student’s unpaired 2-talied t test. *p < 0.05 and **p < 0.01.
Dihydroartemisinin treatment accelerated wound healing in diabetic mice
Full-thickness skin wounds were created on the backs of diabetic mice treated with dihydroartemisinin. Figure 2A displays representative photos of the wound region in each group at 0, 3, 5, 7, 10, 12, and 14 d following surgery. The results revealed that on postoperative day 3, there were no notable variations in all groups. The nondiabetic group had significantly earlier wound closure beginning on day 5, with 31.7% closure versus 19.3 and 7.9% closure in the streptozotocin diabetic + dihydroartemisinin treatment group and the streptozotocin diabetic group, respectively (p < 0.05). Wound healing significantly improved from day 7 to day 14 with an average of 40.5 and 93.8% wound closure in the nondiabetic group versus 34.2 and 91.0% for the streptozotocin diabetic + dihydroartemisinin treatment group and versus 20.2 and 47.1% for the streptozotocin diabetic group, respectively (p < 0.01) (Figure 2B).
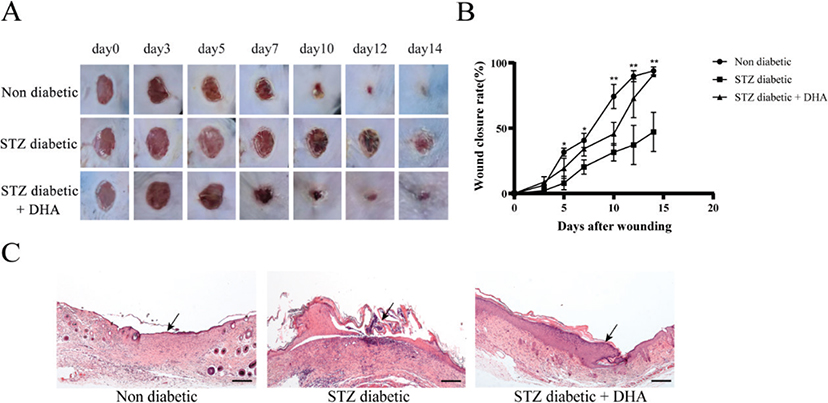
Figure 2. Topical dihydroartemisinin accelerates wound closure in streptozotocin diabetic mice. (A) Days 0, 3, 5, 7, 10 and 14 representative photos of the wound surface in each group. (B) At the indicated time points, the wound areas were measured, and wound closures (the percentage of wound that healed) were calculated (n ≥ 3). The results are expressed as mean ± SD. P values were calculated using the student’s unpaired 2-talied t test. *p < 0.05 and **p < 0.01 for nondiabetic and streptozotocin diabetic + dihydroartemisinin vs. streptozotocin diabetic. (C) Representative histological sections of hematoxylin and eosin staining (scale bar = 200 μm). Arrows indicate epithelialization. The nondiabetic group tended to be normal.
Dihydroartemisinin treatment promoted re-epithelialization
Mice in the nondiabetic group showed complete re-epithelialization and well-formed granulation tissue, as observed by hematoxylin and eosin staining (Figure 2C). Compared with the diabetic group, dihydroartemisinin-treated wounds showed noticeably higher re-epithelialization. These findings imply that one of the dihydroartemisinin’s mechanisms of action is to promote re-epithelialization.
Dihydroartemisinin treatment promoted angiogenesis
Angiogenesis is essential for wound healing because it requires the healing process to transport oxygen and nutrients through the blood into new tissue formation sites. First, CD31 is found on the surface of endothelial cells and is widely used as a marker of angiogenesis. Second, Vascular Endothelial Growth Factor A (VEGFA) is known for its role in directing endothelial cell migration and vascularization during wound healing. We used CD31 immunohistochemistry to identify blood vessels in wound sections and ImageJ’s built-in digital image analysis tools to measure blood vessel density and examine wound bed vascularization. Immunohistochemistry staining for CD31 showed that the mice in the nondiabetic group had more mature blood capillaries in their dermal defects from postoperative day 14. In contrast, the number of new vessels in the streptozotocin diabetic group was relatively low (Figure 3A). The number of mature vessels in the defects treated with dihydroartemisinin was noticeably higher than that in the untreated wounds 14 days after the operation.
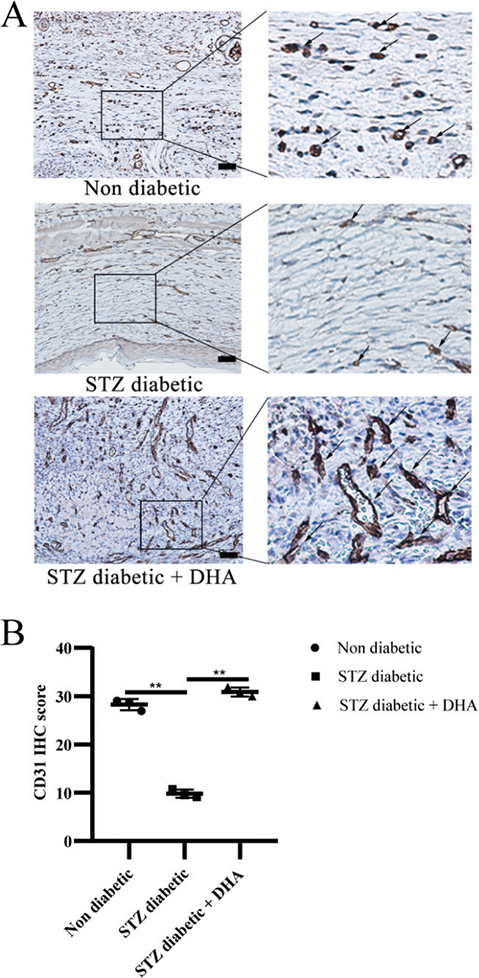
Figure 3. Topical dihydroartemisinin increases microvascular density in wound skin tissues of diabetic mice. (A) The expression of CD31 protein in the wounds of mice was detected by immunohistochemistry. Representative immunohistochemistry images are shown (scale bar = 50 μm). (B) CD31 quantification results are expressed as mean ± SD. P values were calculated using the student’s unpaired 2-talied t test. **p < 0.01.
In addition, CD31 protein expression in the skin of mice was detected by Western blot after 14 days of dosing. As shown in Figure 4A, the streptozotocin diabetic + dihydroartemisinin treatment group had a higher expression of this protein than the streptozotocin diabetic group and the nondiabetic group. This finding supports the immunohistochemistry findings and suggests that topical dihydroartemisinin may encourage neovascularization.
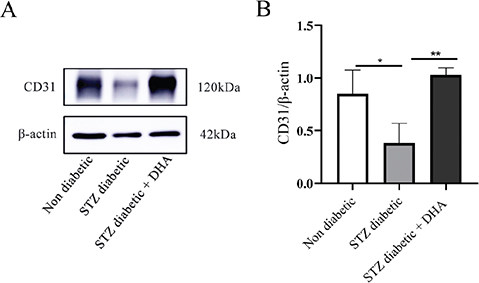
Figure 4. Topical dihydroartemisinin increases CD31 protein level in wound skin tissues of diabetic mice. (A) Immunoblots of CD31 and actin in wound skin tissues of the mice (each lane contains wound skin tissue lysates from an individual mouse). (B) CD31 protein expression in the nondiabetic group vs. the streptozotocin diabetic group vs. the streptozotocin diabetic + dihydroartemisinin group (n ≥ 3). The results are expressed as mean ± SD. P values were calculated using the student’s unpaired 2-talied t test. *p < 0.05 and **p < 0.01.
Angiogenesis is a multistep and complex process regulated by a variety of cytokines and growth factors, among which VEGF is the most important stimulating factor. VEGF induces endothelial cells growth and migration, and prevents its apoptosis. In addition, it enhances vascular permeability in numerous tissues. Next, immunohistochemistry analysis of VEGF was performed to further confirm whether dihydroartemisinin promotes angiogenesis. The findings demonstrated that the positive expression of VEGF in the streptozotocin diabetic + dihydroartemisinin treatment group increased compared with the streptozotocin diabetic group and tended to reach or was greater than that in the nondiabetic group (Figure 5A).
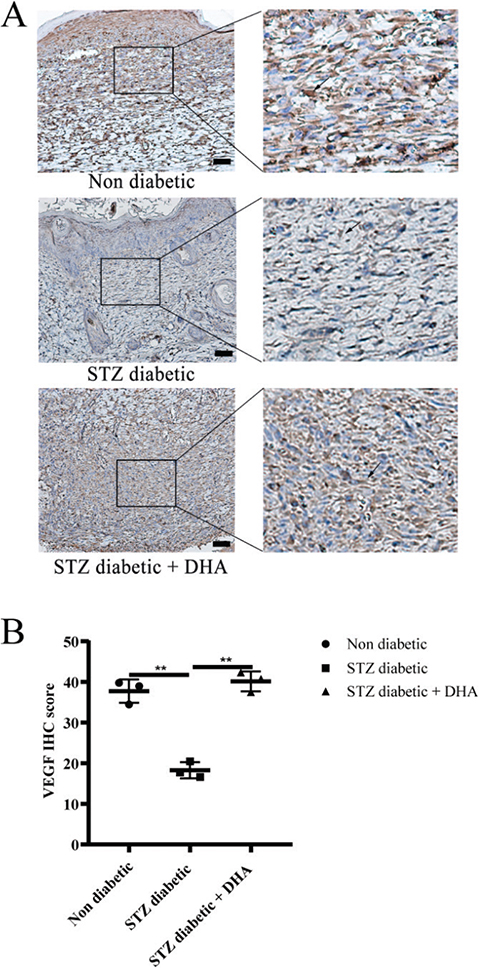
Figure 5. Topical dihydroartemisinin increases vascular endothelial growth factor (VEGF) expression in wound skin tissues of diabetic mice. (A) IHC of VEGF protein on mouse wound skin tissues. Representative immunohistochemistry images are shown (scale bar = 50 μm). (B) VEGF quantification results are expressed as mean ± SD. P values were calculated using the student’s unpaired 2-talied t test. **p < 0.01. Arrows indicate positive expression.
According to the aforementioned findings, dihydroartemisinin therapy accelerate the healing of diabetic wounds. The mechanism of action of VEGF was also examined to determine whether topical dihydroartemisinin improved angiogenesis in diabetic wounds. VEGF expression in diabetic wounds is shown in Figure 6A. According to Western blot results, the streptozotocin diabetic + dihydroartemisinin therapy group and nondiabetic group had considerably higher levels of VEGF total protein than the streptozotocin diabetic group. When VEGF was investigated using Qualitative real-time polymerase chain reaction (qRT-PCR), the findings showed that VEGF expression levels in the dihydroartemisinin treatment group were signficantly higher than those in the stretozotocin diabetic group (Figure 6C). Collectively, these findings suggest that topical dihydroartemisinin may promote angiogenesis and accelerate wound healing.
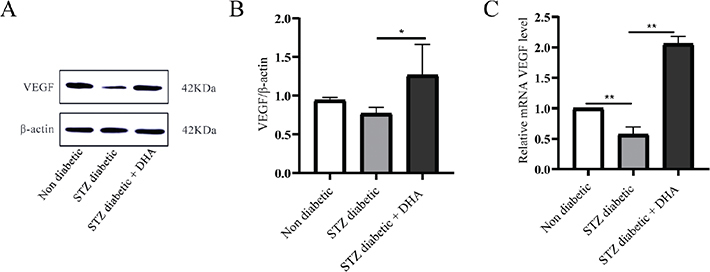
Figure 6. Topical dihydroartemisinin increases the mRNA and protein levels of vascular endothelial growth factor (VEGF) in wound skin tissues of diabetic mice. (A) Immunoblots of VEGF and actin using mouse wound skin tissues (each lane contains wound skin tissue lysates from an individual mouse). (B) VEGF protein expression in the streptozotocin diabetic group vs the streptozotocin diabetic + dihydroartemisinin group (n ≥ 3). (C) Relative mRNA levels of VEGF were measured by qualitative real-time polymerase chain reaction (qRT-PCR). The results are expressed as mean ± SD. P values were calculated using the student’s unpaired 2-talied t test. *p < 0.05 and **p < 0.01.
Discussion
In the present study, topical dihydroartemisinin significantly the time to wound healing and promotes epithelialization at the wound site in diabetic animals. Topical dihydroartemisinin also increased the expression of CD31 and VEGF in the streptozotocin diabetic mice. Therefore, our findings suggest that dihydroartemisinin stimulates angiogenesis by elevating VEGF expressions, and Dihydroartemisinin is typically administered orally or through the digestive tract, where it inhibits tumor cell proliferation and angiogenesis [31,32]. It has been demonstrated to encourage angiogenesis in the early stages of zebrafish embryonic development and is administered by adding the medication directly to systemic water, followed by continuous embryonic culture [17]. In addition, topical dihydroartemisinin has been shown to have positive anti-inflammatory effects, alleviates the signs and symptoms of atopic dermatitis [21], and reduces psoriatic skin inflammation [33,34]. This shows that the various effects of dihydroartemisinin may be related to the various experimental settings and drug delivery methods. As it is unknown how topical dihydroartemisinin affects skin angiogenesis in diabetes, we performed tests on mice that had received streptozotocin injections to confirm the effect of dihydroartemisinin on the skin.
On the one hand, dihydroartemisinin produces few adverse side effects [35]. Topical dihydroartemisinin has therapeutic effect on immune-related diseases, such as atopic dermatitis and psoriasis in mice, thus, topical dihydroartemisinin could become a useful therapy for diabetic wound. On the other hand, dihydroartemisinin inhibited the expression of VEGF in cancer cells, and this reaction mainly occurs in the blood [36]. However, skin topical administration mainly occurs outside the blood vessel. And it is unknown how topical dihydroartemisinin affects skin angiogenesis in diabetes. In addition, DHA have a positive role in angiogenesis in zebrafish [17]. Here, we found that topical dihydroartemisinin increased the expression of CD31 and VEGF in the streptozotocin diabetic mice. Therefore, our findings are consistent to the evidences provided by [17].
In this study, we found that topical DHA dramatically improves the healing of the wound in diabetic mice and increases the expression of CD31 and VEGF. These results indicated that topical dihydroartemisinin stimulates angiogenesis by elevating CD31 and VEGF expression.
Conclusions
The animal tests in our study revealed that topical dihydroartemisinin can boost CD31 and VEGF expression to improve angiogenesis and help diabetic wounds heal.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (82171318), the Academic Program of Shandong First Medical University (2019QL014), and the Shandong Taishan Scholarship (J.L.).
Data availability
The data used to support this study will be made available by the corresponding author upon request.
References
[1] Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
[2] Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–51.
[3] Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43.
[4] Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22.
[5] Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7(35).
[6] Kaushik K, Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21(11):1137–50.
[7] Barnes RC. Point: Hyperbaric oxygen is beneficial for diabetic foot wounds. Clin Infect Dis. 2006;43(2):188–92.
[8] Tsang MW, Wong WKR, Hung CS, Lai KM, Tang W, Cheung EYN, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26(6):1856–61.
[9] Macura M, Frangez HB, Cankar K, Finzgar M, Frangez I. The effect of transcutaneous application of gaseous CO(2)on diabetic chronic wound healing-A double-blind randomized clinical trial. International Wound Journal. 2020;17(6):1607–14.
[10] Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric Oxide Therapy for Diabetic Wound Healing. Adv Healthc Mater. 2019;8(12):e1801210.
[11] Tellechea A, Bai S, Dangwal S, Theocharidis G, Nagai M, Koerner S, et al. Topical Application of a Mast Cell Stabilizer Improves Impaired Diabetic Wound Healing. J Invest Dermatol. 2020;140(4):901–11 e11.
[12] Wang T, He R, Zhao J, Mei JC, Shao MZ, Pan Y, et al. Negative pressure wound therapy inhibits inflammation and upregulates activating transcription factor-3 and downregulates nuclear factor-kappaB in diabetic patients with foot ulcerations. Diabetes Metab Res Rev. 2017;33(4).
[13] Wang CG, Wang M, Xu TZ, Zhang XX, Lin C, Gao WY, et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9(1):65–76.
[14] Chen TY, Wen TK, Dai NT, Hsu SH. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials. 2021;269:120608.
[15] Wang SJ, Sun B, Cheng ZX, Zhou HX, Gao Y, Kong R, et al. Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NF-kappa B pathway. Cancer Chemoth Pharm. 2011;68(6):1421–30.
[16] Chen HH, Zhou HJ, Wang WQ, Wu GD. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemoth Pharm. 2004;53(5):423–32.
[17] Ba Q, Duan J, Tian JQ, Wang ZL, Chen T, Li XG, et al. Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish. Acta Pharmacol Sin. 2013;34(8):1101–7.
[18] Jiang M, Zhong G, Zhu Y, Wang L, He Y, Sun Q, et al. Retardant effect of dihydroartemisinin on ulcerative colitis in a JAK2/STAT3-dependent manner. Acta Biochim Biophys Sin (Shanghai). 2021;53(9):1113–23.
[19] Liu X, Lu J, Liao Y, Liu S, Chen Y, He R, et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed Pharmacother. 2019;117:109070.
[20] Zhang T, Zhang Y, Jiang N, Zhao X, Sang X, Yang N, et al. Dihydroartemisinin regulates the immune system by promotion of CD8(+) T lymphocytes and suppression of B cell responses. Sci China Life Sci. 2020;63(5):737–49.
[21] Xue X, Dong Z, Deng Y, Yin S, Wang P, Liao Y, et al. [Dihydroartemisinin alleviates atopic dermatitis in mice by inhibiting mast cell infiltration]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(10):1480–7.
[22] Du F, Wang NJ, Qiu L, Zhang B, Liang BW, Ye ZG. Study on the methodology of transdermal delivery of dihydroartemisinin in vitro. Journal of Pharmacy of people’s Liberation Army. 2008(05):405–7.
[23] Umehara T, Mori R, Mace KA, Murase T, Abe Y, Yamamoto T, et al. Identification of Specific miRNAs in Neutrophils of Type 2 Diabetic Mice: Overexpression of miRNA-129-2-3p Accelerates Diabetic Wound Healing. Diabetes. 2019;68(3):617–30.
[24] Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–68.
[25] Ning J, Zhao H, Chen B, Mi EZ, Yang Z, Qing W, et al. Argon Mitigates Impaired Wound Healing Process and Enhances Wound Healing In Vitro and In Vivo. Theranostics. 2019;9(2):477–90.
[26] Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020;11(1):4678.
[27] Huang W, Jiao J, Liu J, Huang M, Hu Y, Ran W, et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020;6:84.
[28] Long M, Cai L, Li W, Zhang L, Guo S, Zhang R, et al. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes. 2018;67(3):518–31.
[29] Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120(12):4207–19.
[30] Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, et al. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52(11):2805–13.
[31] Dai X, Zhang X, Chen W, Chen Y, Zhang Q, Mo S, et al. DihydroartemisinIn: A Potential Natural Anticancer Drug. Int J Biol Sci. 2021;17(2):603–22.
[32] Yu R, Jin G, Fujimoto M. DihydroartemisinIn: A Potential Drug for the Treatment of Malignancies and Inflammatory Diseases. Front Oncol. 2021;11:722331.
[33] Chen Y, Yan Y, Liu H, Qiu F, Liang CL, Zhang Q, et al. Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8(+) T-cell memory in wild-type and humanized mice. Theranostics. 2020;10(23):10466–82.
[34] Fan M, Li Y, Yao C, Liu X, Liu J, Yu B. DC32, a Dihydroartemisinin Derivative, Ameliorates Collagen-Induced Arthritis Through an Nrf2-p62-Keap1 Feedback Loop. Front Immunol. 2018;9:2762.
[35] Cheng Z, Qi R, Li L, Liu Q, Zhang W, Zhou X, et al. Dihydroartemisinin ameliorates sepsis-induced hyperpermeability of glomerular endothelium via up-regulation of occludin expression. Biomed Pharmacother. 2018;99:313–8.
[36] Lee J, Zhou HJ, Wu XH. Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia K562 cells. Cancer Chemother Pharmacol. 2006;57(2):213–20.