ORIGINAL ARTICLE
Fresh human amniotic membrane wrapping promotes peripheral nerve regeneration in PGA-collagen tubes
Atsuhiko Iwaoa,b, Hiroto Saijoa, Takafumi Nakayamac, Akihito Higashia,b, Kazuya Kashiyamaa,b, Norisato Mitsutaked and Katsumi Tanakaa,b
aDepartment of Plastic and Reconstructive Surgery, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan; bDepartment of Plastic and Reconstructive Surgery, Nagasaki University Hospital, Nagasaki, Japan; cDepartment of Tumor and Diagnostic Pathology, Nagasaki University, Nagasaki, Japan; dDepartment of Radiation Medical Science, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan
Background: An artificial nerve conduit can interpose the peripheral nerve defect without donor site morbidity. However, treatment outcomes are often unsatisfactory. Human amniotic membrane (HAM) wrapping has been reported to promote peripheral nerve regeneration. We evaluated the effects of a combined application of fresh HAM wrapping and a polyglycolic acid tube filled with collagen (PGA-c) in a rat sciatic nerve 8-mm defect model.
Methods: The rats were divided into three groups: (1) the PGA-c group (n = 5), in which the gap was interposed with the PGA-c; (2) the PGA-c/HAM group (n = 5), in which the gap was interposed with the PGA-c bridge, then HAM (14 × 7 mm) was wrapped around it; and (3) the Sham group (n = 5). Walking-Track recovery, electromyographic recovery, and histological recovery of the regenerated nerve were evaluated at 12 weeks postoperatively.
Results: Compared to the PGA-c group, the PGA-c/HAM group showed significantly better recovery in terminal latency (3.4 ± 0.31 ms vs. 6.6 ± 0.72 ms, p < 0.001), compound muscle action potential (0.19 ± 0.025 mV vs. 0.072 ± 0.027 mV, p < 0.01), myelinated axon perimeter (15 ± 1.3 µm vs. 8.7 ± 0.63 µm, p < 0.01), and g-ratio (0.69 ± 0.0089 vs. 0.78 ± 0.014, p < 0.001).
Conclusion: This combined application highly promotes peripheral nerve regeneration and may be more useful than PGA-c alone.
KEYWORDS: Peripheral nerve regeneration; rat sciatic nerve defect model; human amniotic membrane; PGA-collagen tube
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 13–17. DOI: https://doi.org/10.2340/jphs.v58.6496.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 20 December 2022; Accepted: 28 February 2023. Published: 2023-05-23.
CONTACT Atsuhiko Iwao a-iwao@nagasaki-u.ac.jp 1-7-1, Sakamoto, Nagasaki-shi, Nagasaki, 852-8102, Japan
Competing interests and funding: The authors report there are no competing interests of declare.
Introduction
Peripheral nerve defects often result from trauma or surgery for a malignant tumor in clinical practice. An autologous nerve graft is the first choice for the reconstruction of peripheral nerve defects. However, it may cause donor site morbidities such as hypoesthesia, numbness, and pain caused by amputated neuroma. Although an artificial nerve conduit has the advantage of no donor site morbidities, treatment outcomes are inferior to those of autologous nerve graft [1,2]. This is because the conduit only acts as scaffolds for nerve regeneration, while cells, growth factors, and scaffolds are generally necessary for efficient tissue regeneration. Thus, various types of stem cells [3] or growth factors [4] have been used with the artificial nerve conduit to enhance the nerve regeneration potential.
In recent years, a technique in which a small fragment cut from human amniotic membrane (HAM) was wrapped around the injured nerve has been reported in a rat sciatic nerve injury model for the purpose of promoting peripheral nerve regeneration. HAM is the innermost layer of the placenta and enwraps the fetus in the amniotic cavity. It consists of two layers: the amnion and the chorion. This avascular tissue contains amniotic epithelial cells and amniotic mesenchymal stem cells [5]. In addition, HAM is rich in growth factors such as basic fibroblast growth factor and transforming growth factor α [6]. Previous studies have reported that fresh HAM wrapping reduced scar formation, which inhibited axonal regeneration, and promoted peripheral nerve regeneration in rat sciatic nerve adhesion models [7,8].
In the treatment of peripheral nerve defects, nerve regeneration may be further promoted by adding HAM-containing cells and growth factors to the artificial nerve conduit. The purpose of this study was to investigate the effect of a combined application using fresh HAM wrapping and a polyglycolic acid tube filled with collagen (PGA-c) for a rat sciatic nerve defect model.
Material and methods
Animals
This study was approved by Biomedical Research Center of Nagasaki University (approval number: 2005271634). A total of 15 Sprague Dawley rats (6-week-old males) weighing 180–225 g (Japan SLC) were used. All invasive procedures were performed under anesthesia with intraperitoneal administration of medetomidine (0.375 mg/kg), midazolam (2 mg/kg), and butorphanol (0.25 mg/kg). At the end of this study, all rats were sacrificed with the use of carbon dioxide.
HAM preparation
Fresh human placenta was collected immediately after cesarean section in accordance with the Declaration of Helsinki and with the approval of the Institutional Ethics Committee. Informed consent had been obtained from the volunteer at the Amniotic Membrane Bank in Nagasaki University Hospital in advance. This placenta was from a woman with a history of laparoscopic cervical cerclage but no history of infectious disease such as hepatitis, HIV, and venereal disease. HAM was manually removed from the placenta. All blood clots and debris were removed by washing the tissue with saline. The HAM was cut into 14 × 7 mm fragments and then washed with phosphate-buffered saline (PBS) for 30 min three times. The HAM was transferred into a rat sciatic nerve defect model within 24 h after delivery.
Surgical procedure
The right sciatic nerves of rats were exposed under general anesthesia. The nerves were sufficiently dissected in the area between the sciatic notch and its bifurcation. The animals were randomly divided into three groups: (1) PGA-c group (n = 5), where 8 mm of the sciatic nerve was removed and a 10 mm PGA-c (Nerbridge®, Toyobo, Japan) bridged the gap; (2) PGA-c/HAM group (n = 5), where 8 mm of the sciatic nerve was removed and a 10 mm PGA-c bridged the gap, then HAM (14 × 7 mm) was wrapped around it; and (3) Sham group (n = 5), where only the sciatic nerve was dissected. The distal and proximal sciatic nerve stumps were pulled 1 mm into each side of the PGA-c tube and sutured with two horizontal mattress sutures using 10-0 nylon. In the PGA-c/HAM group, HAM was wrapped around the PGA-c tube so that the chorion side was in contact with the tube, then HAM was sutured to itself at four points (Figure 1(a), 1(b)). Finally, the skin was closed with 4-0 nylon sutures. The same surgeon performed all procedures.
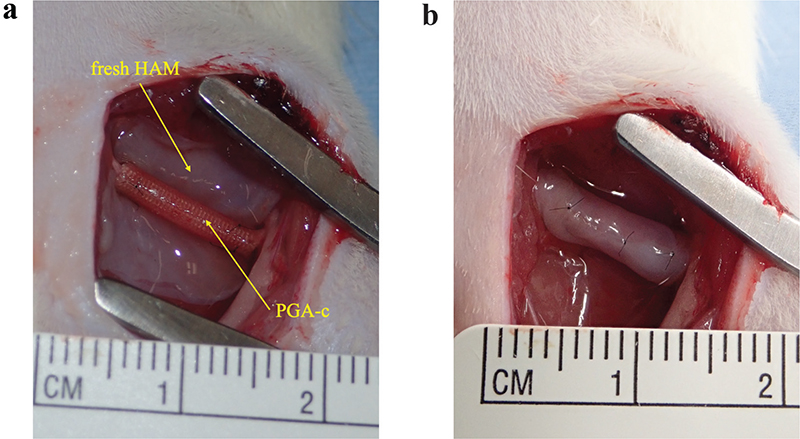
Figure 1. Human amniotic membrane (HAM) preparation and surgical procedure. Fresh HAM was delivered from a healthy woman. After washing and making into 14 mm × 7 mm fragments (a), the HAM was wrapped around a 10-mm polyglycolic acid tube filled with collagen (PGA-c) bridging 8-mm sciatic nerve defect (b).
Walking-track assessment
Footprints of each rat hind paw while walking were taken at 12 weeks after surgery. The toe spread (TS), intermediate toe spread (ITS), and paw length (PL) of both limbs (E: experimental side; N: normal side) were measured; then the sciatic functional index (SFI) was calculated using the following formula [9]:
SFI = –38.1 × (EPL – NPL) / NPL + 109.5 × (ETS – NTS) / NTS + 13.3 × (EITS – NITS) / NITS – 8.8
Electromyographic assessment
Electromyographic analysis was performed at 12 weeks postoperation. Bipolar stimulating electrodes were placed under the sciatic nerve 5 mm proximal to the PGA-c. The compound muscle action potential (CMAP) and terminal latency (TL) were recorded using needle electrodes inserted into the middle of the tibialis anterior muscle and its tendon using an electromyogram (USE-100, UNIQUE MEDICAL, Japan).
Histological assessment of the nerve
Rats were sacrificed at 12 weeks postoperatively. The sciatic nerve, including the nerve regeneration site, was harvested at a length of 10 mm. Specimens were soaked in 2.5% glutaraldehyde as prefixation and then soaked in 2% osmium tetroxide as postfixation. Samples were dehydrated using serial ethanol dilutions (50, 70, 80, 90, 95, 99.5, and 100%). Ultra-thin (80-nm) sections at the midpoint of the regeneration site transversally were impregnated with epoxy resin. These sections were observed under a transmission electron microscope (TEM) (JEM-1200EX, JEOL, Japan). Five random fields of each nerve at 1000× magnification were selected and observed by an observer who was blinded to the treatment groups. In addition, 20 myelinated axons were selected from each field. The myelinated axon perimeter was measured using the ImageJ software (National Institute of Health), and g-ratio (axon perimeter/fiber perimeter) was calculated. These indicators represented the promotion of myelination.
Statistical assessment
Experimental data were expressed as means ± standard error and analyzed using one-way analysis of variance (ANOVA). To detect difference between groups, data were analyzed using the Tukey post-hoc test. p-Values of < 0.05 were defined as significant. The statistical analyses were carried out with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [10].
Results
Walking-track recovery
First, we calculated the SFI to evaluate motor function recovery. An SFI of 0 represents normal, and −100 represents complete dysfunction. Compared to the Sham group, SFIs of both the PGA-c and PGA-c/HAM groups were far lower. No significant difference was observed between the PGA-c group and the PGA-c/HAM group (−78 ± 6.7 vs. −69 ± 7.6, p = 0.59). This suggests that there was no major difference in functional recovery between the two groups (Figure 2).
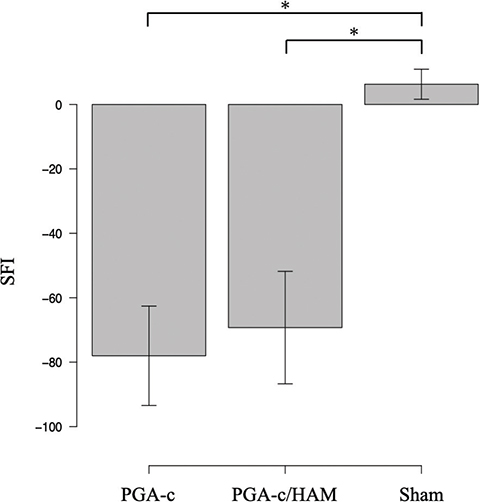
Figure 2. Walking-track recovery was evaluated by the sciatic functional index (SFI) value at 12 weeks postoperatively. Each bar represents mean and standard error of five animals. *p < 0.05. HAM: human amniotic membrane; PGA-c: polyglycolic acid tube filled with collagen.
Electromyographic recovery
Next, we performed electromyographic assessments. TL in the PGA-c/HAM group was significantly shorter than that in the PGA-c group (3.4 ± 0.31 ms vs. 6.6 ± 0.72 ms, p < 0.001), but they were longer than that in the Sham group (1.4 ± 0.058 ms) (Figure 3(a)). Simultaneously, CMAP in the PGA-c/HAM group was significantly higher than that in the PGA-c group (0.19 ± 0.025 mV vs. 0.072 ± 0.027 mV, p < 0.01) although it did not reach that in the Sham group (0.32 ± 0.013 mV) (Figure 3(b)). These results indicate that the combination of PGA-c and HAM promotes significantly better electromyographic recovery than PGA-c alone.
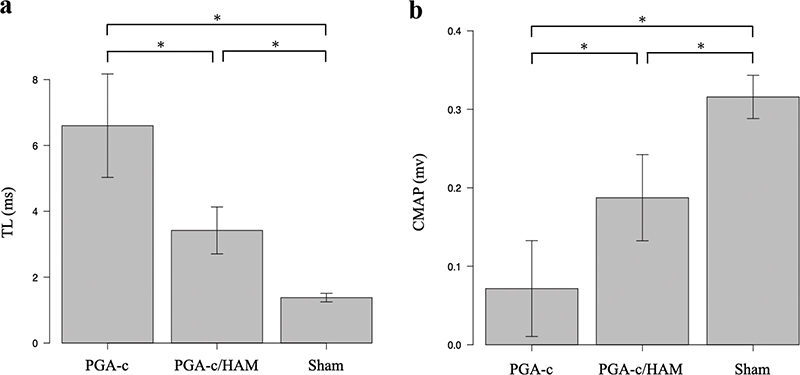
Figure 3. Electromyographic recovery at 12 weeks postoperatively. Terminal latency (TL) (a) and compound muscle action potential (CMAP) (b) of each group are shown. Each bar represents mean and standard error of five animals. *p < 0.05. HAM: human amniotic membrane; PGA-c: polyglycolic acid tube filled with collagen.
Histological recovery of the nerve
We performed histomorphometric assessments of the regenerated nerve using a TEM (Figure 4(a–c)). The myelinated axon perimeter in the PGA-c/HAM group was significantly longer than that in the PGA-c group (15 ± 1.3 µm vs. 9.8 ± 0.39 µm, p < 0.01) (Figure 4(d)), but they were shorter than that in the Sham group (33 ± 0.94 µm). Next, g-ratio, which is a highly reliable indicator for assessing myelination, was calculated. A g-ratio between 0.6 and 0.7 indicates quality and maturation of myelin sheath. The g-ratio in the PGA-c/HAM group was significantly lower than that in the PGA-c group (0.69 ± 0.0089 vs. 0.78 ± 0.014, p < 0.001) (Figure 4(e)), although they were still higher than that in the Sham group (0.058 ± 0.0085). The distribution of myelinated axon perimeter in the PGA-c/HAM group was shifted toward longer compared with the PGA-c group (Figure 4(f)).
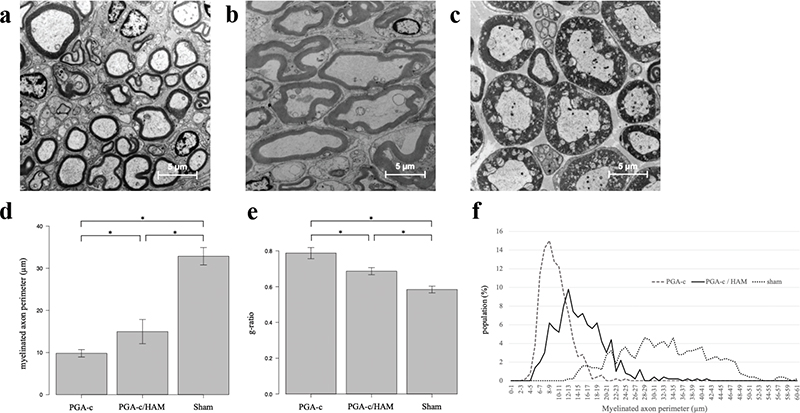
Figure 4. Histomorphometric results of the regenerated sciatic nerve at 12 weeks postoperatively. transmission electron microscope (TEM) photographs showing cross-sectional slices of the regenerated sciatic nerve in the polyglycolic acid tube filled with collagen (PGA-c) (a), PGA-c/HAM (human amniotic membrane) (b), and Sham groups (c); scale bar = 5 µm. Myelinated axon perimeters (d). g-ratio (e). Each bar represents mean and standard error of five animals. *p < 0.05 (d and e). The distribution of longer myelinated axon perimeters (f).
Discussion
This study evaluated the effect of fresh HAM on PGA-c in peripheral nerve regeneration using a rat sciatic nerve defect model. Our results showed that fresh HAM wrapping significantly recovered myelinated axon perimeter and g-ratio. These findings suggested that fresh HAM wrapping would contribute to axonal maturation and myelination during peripheral nerve regeneration. In addition, this histological recovery of the nerve may have contributed to the significantly better neuromuscular recovery as represented by the TL value and the CMAP value. However, no significantly better recovery was observed in the PGA-c/HAM group compared to the PGA-c group with regard to SFI.
Several studies reported the effectiveness of amnion or amniotic membrane application for rat sciatic nerve defect models. An experiment using frozen human amnion processed into a tubular form showed better axonal regeneration than a silicone tube in a rat sciatic nerve 10-mm defect model [11]. However, the amnion tube has difficulty in maintaining its tubular formation due to its flexibility and lack of rigidity. To avoid collapsing, the amnion muscle combined graft (AMCG) conduit was invented [12]. The AMCG conduit was created by the insertion of skeletal muscle into the lumen of dehydrated amnion tube, and it showed a good peripheral nerve recovery in a rat 15-mm median nerve defect model. However, the recovery with respect to grasping test, myelinated axon diameter, and g-ratio did not reach that observed in the autograft. The AMCG conduit may also cause donor muscle morbidity. In addition, these studies have not been compared to commercially available artificial nerve conduit. For the sake of applying HAM to the nerve defect, we devised a new method of wrapping the HAM around an existing artificial nerve conduit from the outside. Then, HAM wrapping was verified whether it improved the ability of the conduit.
We consider that fresh HAM is the optimal condition for the application of HAM wrapping in the treatment of peripheral nerve defect because fresh HAM contains more stem cells with higher viability and richer amounts of angiogenic growth factors than preserved HAM [13]. An experiment evaluating the effect of cryopreserved HAM wrapping around autologous nerve transplantation in a rat 10-mm sciatic nerve defect model showed significantly less adhesion to the nerve and less scar formation than controls, but functional and morphological recovery was not improved [14]. Although the extracellular matrix (ECM) of HAM suppressed TGF-ß signaling [15] and reduced scar formation, which impeded peripheral nerve regeneration [7], the effect of ECM alone may have been insufficient for nerve regeneration in a refractory model such as the nerve defect model. Human amniotic mesenchymal stem cells (hAMSCs) are abundant in fresh HAM [5,16]. It was reported that hAMSCs administrated intracerebrally survived for at least 27 days and significantly increased the expression of brain-derived neurotrophic factor (BDNF) in a rat intracerebral hemorrhage model. As a result, neurogenesis was promoted [17]. BDNF acts through the neurotrophin receptor p75NTR to enhance myelin formation in vitro [18,19]. We hypothesize that BDNF secreted from hAMSCs within the HAM may have promoted peripheral nerve regeneration in this experiment.
There is a limitation in this study. First, this procedure requires implantation in the nerve defect immediately after fresh HAM delivery because there is no effective way to preserve it at the present time. Thus, the timing of peripheral nerve surgery needs to coincide with the timing of cesarean section. This combined method is difficult to use in emergency operations such as trauma cases. Further research on preservation methods to maximize cell viability and amount of growth factors in fresh HAM is necessary. Second, we could not demonstrate the significant functional recovery in SFI. The neuromuscular system may still be in the process of recovery at 12 weeks postoperatively. Longer observation periods could resolve this issue.
This study demonstrated that a combination of fresh HAM wrapping and PGA-c promotes peripheral nerve regeneration better than PGA-c alone in several neurological assessments.
Acknowledgements
I would like to thank Specially Appointed Professor Hiroyuki Tanaka in Department of Orthopedic Surgery, Osaka University Graduate School of Medicine for useful advice on how to proceed and framework our research. I am also grateful to Associate Professor Kazuhiro Nagai in Transfusion and Cell Therapy Unit, Nagasaki University Hospital for the donation of amniotic membrane.
Funding
This study was supported by JSPS KAKENHI (Grants-in-Aid for Young Scientists) Grant Number JP 20K18414.
References
[1] Longo MV, Marques de Faria JC, Issac C, et al. Comparisons of the results of peripheral nerve defect repair with fibrin conduit and autologous nerve graft: an experimental study in rats. Microsurgery. 2016;36(1):59–65. https://doi.org/10.1002/micr.22413
[2] Saitzman EB, Villa JC, Doty SB, et al. A comparison between two collagen nerve conduits and nerve autograft: a rat model of motor nerve regeneration. J Hand Surg Am. 2019;44(8):700.e1–700.e9. https://doi.org/10.1016/j.jhsa.2018.10.008
[3] Sowa Y, Kishida T, Imura T, et al. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plast Reconstr Surg. 2016;137(2):318e–330e. https://doi.org/10.1097/01.prs.0000475762.86580.36
[4] Fadia NB, Bliley JM, DiBernardo GA, et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci Transl Med. 2020;12(537):eaav7753. https://doi.org/10.1126/scitranslmed.aav7753
[5] Wassmer CH, Berishvili E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regeneration medicine. Curr Diab Rep. 2020;20(8):31. https://doi.org/10.1007/s11892-020-01316-w
[6] Koob TJ, Lim JJ, Zabek N, et al. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater. 2015;103(5):1133–1140. https://doi.org/10.1002/jbm.b.33265
[7] Atkins S, Smith KG, Loescher AR, et al. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport. 2006;17:1245–1249. https://doi.org/10.1097/01.wnr.0000230519.39456.ea
[8] Lemke A, Ferguson J, Gross K, et al. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomater. 2018;66:335–349. https://doi.org/10.1016/j.actbio.2017.11.042
[9] Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83(1):129–136. https://doi.org/10.1097/00006534-198901000-00024
[10] Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. https://doi.org/10.1038/bmt.2012.244
[11] Mohammand J, Shenaq J, Rabinovsky E, et al. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Sug. 2000;105(2):660–666. https://doi.org/10.1097/00006534-200002000-00027
[12] Marchesini A, Raimondo S, Zingaretti N, et al. The amnion muscle combined graft (AMCG) conduits in nerves repair: an anatomical and experimental study on a rat model. J Mete Sci Mater Med. 2018;29:120. https://doi.org/10.1007/s10856-018-6126-5
[13] Wolbank S, Hildner F, Redl H, et al. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med. 2009;3(8):651–654. https://doi.org/10.1002/term.207
[14] Meng H, Li M, You F, et al. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett. 2011;496(1):48–53. https://doi.org/10.1016/j.neulet.2011.03.090
[15] Lee SB, Li DQ, Tan DT, et al. Suppression of TGF- ß signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20(4):325–334. https://doi.org/10.1076/0271-3683(200004)2041-5FT325
[16] Bourgeois M, Loisel F, Obert L, et al. Can the amniotic membrane be used to treat peripheral nerve defect? A review of literature. Hand Surg Rehabil. 2019;38(4):223–232. https://doi.org/10.1016/j.hansur.2019.05.006
[17] Zhou H, Zhang H, Yan Z, et al. Transplantation of human amniotic mesenchymal stem cells promotes neurological recovery in an intracerebral hemorrhage rat model. Biochem Biophys Res Commun. 2016;475(2):202–208. https://doi.org/10.1016/j.bbrc.2016.05.075
[18] Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298(5596):1245–1248. https://doi.org/10.1126/science.1076595
[19] Yamauchi J, Chan JR, Shooter EM. Neuritrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100(24):14421–14426. https://doi.org/10.1073/pnas.2336152100