ORIGINAL RESEARCH ARTICLE
Triple nerve transfers for the management of early unilateral facial palsy
Jose E. Telich-Tarribaa,b, David F. Navarro-Barquina, Genesis Pineda-Aldanaa and Alexander Cardenas-Mejiaa
aPlastic and Reconstructive Surgery Division, Hospital General ‘Dr. Manuel Gea Gonzalez’, Mexico City, Mexico; bPlastic Surgery Department, Hospital Angeles Pedregal, Mexico City, Mexico
ABSTRACT
Background: Early onset facial paralysis is usually managed with cross-face nerve grafts, however the low number of axons that reach the target muscle may result in weakness or failure. Multiple-source innervation, or ‘supercharging’, seeks to combine the advantages of different donor nerves while minimizing their weaknesses. We propose a combination of cross-face nerve grafts with local extra-facial nerve transfers to achieve earlier facial reanimation in our patients.
Methods: A retrospective cohort including all patients with early unilateral facial palsy (<12 months evolution) who underwent triple nerve transfer between 2019 and 2021 was conducted. We performed single-stage procedure including zygomatic-to-zygomatic and buccal-to-buccal cross-face grafts, a nerve-to-masseter to bucozygomatic trunk transfer, and a mini-hypoglossal to marginal branch transfer. Results were evaluated using the clinician-graded facial function scale (eFACE).
Results: Fifteen patients were included (eight females, seven males), mean age at the time of surgery was 48.9 ± 13.3 years. Palsy was right-sided in eight cases. The mean time from palsy onset to surgery was 5.5 ± 2.8 months. Patients showed improvement in static (70.8 ± 21.9 vs. 84.15 ± 6.68, p = 0.002) and dynamic scores (20 ± 16.32 vs. 74.23 ± 7.46, p < 0.001), as well as periocular (57.33 ± 15.23 vs. 74 ± 7.18, p = 0.007), smile (54.73 ± 11.93 vs. 85.62 ± 3.86, p < 0.001), mid-face (46.33 ± 18.04 vs. 95 ± 7.21, p < 0.001) and lower face scores (67.4 ± 1.55 vs. 90.31 ± 7.54, p < 0.001).
Conclusion: The triple nerve transfer technique using cross-face nerve grafts, the nerve-to-masseter, and the hypoglossal nerve, is an effective and reproducible technique to obtain middle and lower face reanimation in cases of early facial palsy.
KEYWORDS: Facial paralysis; nerve transfer; peripheral nerves; nerve-to-masseter; cross-face nerve graft; hypoglossal nerve; facial reanimation
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 62–66. DOI: https://doi.org/10.2340/jphs.v58.6527.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 28 December 2022; Accepted: 02 April 2023; Published: 09 August 2023
CONTACT Alexander Cardenas-Mejia alexcardenas@hotmail.com Plastic and Reconstructive Surgery Division, Hospital General ‘Dr. Manuel Gea Gonzalez’, Postgraduate Division of the Medical, School, Universidad Nacional Autonoma de Mexico, Av Calzada de Tlalpan 4800, Tlalpan, Mexico City, 14080, Mexico.
Competing interests and funding: The authors have no conflicts of interest to declare.
Background
Facial palsy is a condition that can significantly impair function and appearance. The muscles responsible for facial expression are essential for communication, eye protection, nasal breathing, speech, and maintaining oral continence [1].
Several approaches have been developed to restore facial function and aesthetics, with varying levels of success. In early cases of facial palsy reinnervation may be possible as long as the muscle retains viable motor units [2]. Repair of the ipsilateral facial nerve is the ideal strategy for the return of facial function, unfortunately, this may not be possible in patients with intracranial injuries or damage to the proximal nerve stump. Alternative axon sources are required for these scenarios, either from facial or extra-facial sources [3].
Cross-face nerve grafts from the contralateral facial nerve provide synchronic and spontaneous movement but may result in weak contraction or treatment failure due to the number of axons that reach the target muscle. A lengthy recovery period, donor site morbidity, and the need for additional surgical procedures may result in a cumbersome and inconvenient recovery for some patients, as well as increasing overall expenses and the risk for complications [4].
Extra-facial nerve transfers can provide a higher axonal load, resulting in faster recovery and a wider range of motion. However, the movement they generate may not be spontaneous or synchronized with the contralateral side and may result in significant co-contractions when used as a single motor source [5]. The most commonly used extra-facial donors are the nerve-to-masseter and the hypoglossal nerve. Other sources such as the spinal accessory nerve and the ipsilateral seventh cervical root (C7) have also been described as donors, but the potential morbidity associated with their use has rendered them anecdotic [6].
The ideal facial nerve reconstruction should be able to provide strong and spontaneous muscle contractions that respond to emotions. No single nerve source has been shown to achieve this result consistently. As a result, some centers have proposed using multiple neural inputs for facial reanimation. This approach, known as ‘supercharging’ or ‘multiple-source innervation’ seeks to combine the advantages of different donor nerves, while minimizing their weaknesses. A few studies have shown promising results using this approach for mid-facial reanimation, combining cross-face nerve grafts to the zygomatic and buccal branches with the nerve-to-masseter [2, 7–10].
The lower lip has often been neglected in facial reanimation procedures, but its tone is important for achieving oral competence and symmetrical lip function. Adding a mini-hypoglossal nerve transfer to the marginal branch can increase muscle tone at rest and improve results in this area [11].
This work aims to propose a triple transfer technique combining cross-face nerve grafts with nerve-to-masseter and mini-hypoglossal nerve transfers to restore function to the middle and lower thirds of the face (Figure 1).
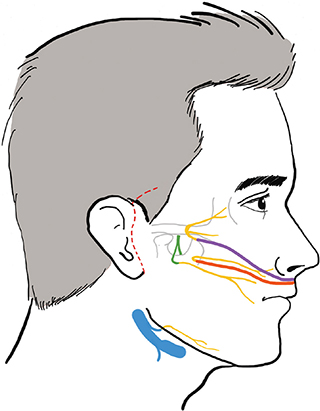
Figure 1. Configuration of the triple nerve transfer.
Methods
We performed a cohort study including all patients with early unilateral facial palsy (<12 months evolution) and neurophysiological evidence of viable motor units who underwent triple nerve transfer between 2019 and 2021. All patients underwent preoperative clinical and neurophysiological evaluation.
Full approval by the institutional ethics board was obtained (Registration number 05-47-2020). Information was recorded using a data sheet including patients’ demographic characteristics, cause of facial paralysis, pre- and post-operative functional assessment using the clinician-graded facial function scale (eFACE) [12].
Descriptive analyses were performed. Continuous variables are expressed in central tendency measures and categorical values are presented as percentages. Comparison between pre- and post-operative variables was done using the Mann–Whitney U test.
Surgical technique
Two surgical teams work simultaneously, one on the face and the second on the legs. A preauricular incision is made on the healthy hemiface and the skin flap is dissected anteriorly until a buccal and zygomatic branch of the facial nerve are identified with help of an electrical stimulator (Figure 2). Branches that achieve pure eye closure and oral commissure excursion are selected and transected.
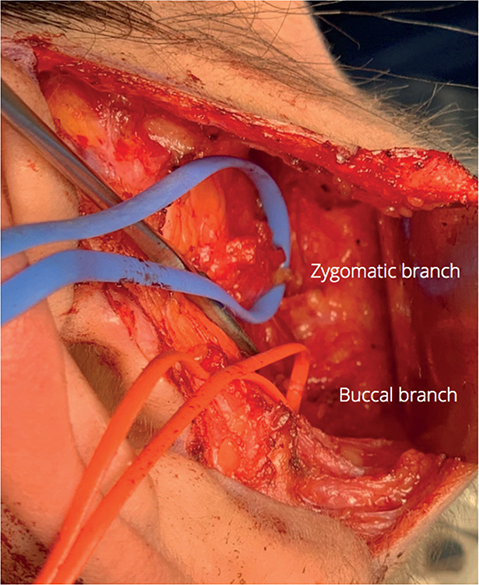
Figure 2. Intraoperative photo showing the buccal and zygomatic branches on the non-paralyzed side.
On the paralyzed face, a preauricular incision with temporal extension is done and the skin flap is raised. The zygomatic branch is located over the zygomatic arch and dissected proximally to identify the bucozygomatic trunk and a distal buccal branch (Figure 3).
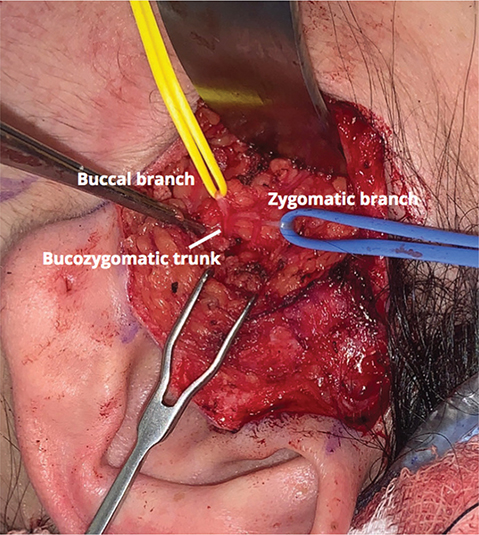
Figure 3. Intraoperative photo showing the buccal and zygomatic branches, as well as the bucozygomatic trunk on the paralyzed side.
The nerve-to-masseter is harvested over the mandibular notch by blunt dissection. A submandibular incision is done to identify the marginal branch and hypoglossal nerve. Two sural nerve grafts are harvested and transferred to the face in an orthodromic fashion. End-to-end anastomoses are done between the buccal branches, the zygomatic branches, and between the nerve-to-masseter to the bucozygomatic trunk, while an end-to-side anastomosis is performed between the marginal and hypoglossal nerves.
Patients were discharged 24 h after surgery, and began physical therapy 4 weeks later.
Results
Fifteen patients (eight females and seven males) with a mean age of 48.9 ± 13.3 years were included. Paralysis was right-sided in eight cases (53.3%) and left in seven (46.6%). Etiology of facial palsy was secondary to acoustic neuromas in five patients (33.3%), parotid surgery in five cases (33.3%), secondary to intracranial surgery in three (20%) and traumatic in two patients (13.3%) (Table 1) .
The mean time from palsy onset to surgery was 5.5 ± 2.8 months. The first signs of facial movement were seen on average 3.3 ± 0.4 months after surgery. Mean follow-up time was 10.4 ± 3.9 months.
eFace results revealed a statistically significant improvement for static (70.8 ± 21.9 vs. 84.15 ± 6.68, p = 0.002) and dynamic scores (20 ± 16.32 vs. 74.23 ± 7.46, p < 0.001), as well as periocular (57.33 ± 15.23 vs. 74 ± 7.18, p = 0.007), smile (54.73 ± 11.93 vs. 85.62 ± 3.86, p < 0.001), mid-face (46.33 ± 18.04 vs. 95 ± 7.21, p < 0.001) and lower face scores (67.4 ± 1.55 vs. 90.31 ± 7.54, p < 0.001). Synkinesis score also had a statistically significant change, however co-contractions were mild in all cases (100 vs. 97.15 ± 2.58, p = 0.006) (Table 2) (Video 1).
Video 1. Case example with 12-month follow-up.
Discussion
Smile restoration surgery has been thoroughly proven to improve the quality of life for individuals with facial paralysis. However, deciding on the best surgical approach for the management of early facial paralysis can be challenging. Neurotization procedures, which involve transferring a nerve to restore function to the mimetic muscles, can be effective as long as there are viable motor endplates available [3].
Early repair of the affected seventh cranial nerve is generally considered the ideal approach for restoring facial function. When this is not feasible, alternative neural sources are required. The traditional focus in facial reanimation surgery has been on reinnervating the zygomatic and buccal branches to restore eye closure and smiling. Cross-face nerve grafts from the contralateral seventh cranial nerve were initially thought to be an ideal option to restore symmetry and spontaneity, unfortunately the low number of axons that reach the motor end plates can limit their effectiveness. Alternatively, extra-facial transfers supply a larger number of axons, are closer and may be easier to harvest, but do not provide the same level of automatism [5, 6].
Facial nerve surgeons have drawn inspiration from techniques used in brachial plexus reconstruction to propose using multiple neural sources to improve reconstructive results in facial palsy. The first reports of combined transfers were made by Terzis, who proposed the ‘babysitter procedure’, which involves combining multiple cross-face nerve grafts with a mini-hypoglossal transfer [13]. Since then, a number of successful dual innervation techniques using various neural sources have been described in the literature [7, 11].
While multiple reanimation techniques have been successful in restoring function to the mid-face, they have often neglected the lower face. The lower lip is an important part of a symmetrical smile, and is also essential for oral continence and avoiding accidental lip biting. Although multiple reanimation techniques have been described for the marginal branch, they tend to be done as secondary procedures [14–16].
The triple transfer technique seeks the complete reanimation of the middle and lower thirds of the face in a single surgical event. Cross-face nerve grafts provide symmetry and spontaneity, the nerve-to-masseter allows for a strong smile, while the hypoglossal nerve supplies a basal tone to the lower lip.
Bigliolli first reported a form of triple nerve transfer for facial reanimation in 2018, with good results [11]. Overall, the neurotization technique differs from ours. In Bigliolli’s series the nerve-to-masseter is connected to the temporofacial nerve trunk, a partial hypoglossal nerve is anastomosed to the cervicofacial trunk, and the cross-face nerve grafts are directed towards the buccal and zygomatic branches. This approach can be seen as a double ‘babysitter procedure’ and in theory may carry an increased risk of synkinesis.
Our configuration is better suited for patients with an early or immediate diagnosis of facial palsy. Neurorraphies are made specifically on the branches we wish to reanimate, thus achieving more targeted and precise results while simplifying rehabilitation. Reinnervation can be successful up to 12 to 18 months after denervation, although there is no defined cutoff limit for denervation, neurophysiological evidence of viable motor end-plates should be obtained before neurotization [5].
One might argue that adding a CFNG to extra-facial transfers could be unnecessary, given that the nerve-to-masseter and hypoglossal nerves have high axonal density and generate robust muscle contractions. Nevertheless, integrating donors from the opposite face enables the involvement of the limbic system and leads to a more natural and sincere smile.
There is an ongoing debate about whether single-stage or two-stage procedures can yield better results for facial reanimation. While a two-stage strategy may allow for more specific branch selection, it can also increase logistical and economic costs for patients and healthcare systems, as well as elevating risks for anesthesia and surgical complications [17]. This becomes even more relevant in systems such as ours, where access to highly specialized treatment is limited and waiting periods for surgery can be prolonged [18, 19].
The main limitations of our study are its small patient sample and its retrospective nature. Nonetheless, it should be observed that referral of patients with early facial paralysis is limited in our institution and this patient population accounts for a fraction of all dynamic facial reconstruction performed by our team [1]. However, we believe using the eFACE app to evaluate our cohort gives strength to our results by being more objective than other scales such as House-Brackman and allowing independent regional evaluation of the different facial thirds. Dynamic scores were improved in all aspects and although periocular synkinesis were identified in some patients upon nerve-to-masseter activation, these were mild and did not cause discomfort for patients. Further research with larger sample sizes, longer follow-up, and prospective study designs may be needed to confirm and expand upon these findings.
Future facial reanimation strategies should focus on achieving global reanimation, including the upper third of the face. Some centers propose innervation by other sources such as the ansa cervicalis or the deep temporal nerve, but the long-term results of these approaches have not yet been reported [20, 21].
Conclusion
The triple nerve transfer technique using cross-face nerve grafts, the nerve-to-masseter, and the mini-hypoglossal nerve, is an effective and reproducible technique to obtain middle and lower face reanimation in cases of early facial palsy.
References
[1] Chávez-Serna E, Telich-Tarriba JE, Altamirano-Arcos C, Nahas-Combina L, Cárdenas-Mejía A. Facial paralysis, etiology and surgical treatment in a tertiary care center in plastic and reconstructive surgery in Mexico. Cir Cir. 2021;89(6):718–727. https://doi.org/10.24875/CIRU.20000916
[2] Kim IA, Maxim T, Echanique K. Modern cross-facial nerve grafting in facial paralysis. Op Tech Otol Head Neck Surg. 2022;33(1):20–28. https://doi.org/10.1016/j.otot.2022.02.004
[3] Garcia RM, Hadlock TA, Klebuc MJ, Simpson RL, Zenn MR, Marcus JR. Contemporary solutions for the treatment of facial nerve paralysis. Plast Reconstr Surg. 2015;135(6):1025e–1046e. https://doi.org/10.1097/PRS.0000000000001273
[4] Jandali D, Revenaugh PC. Facial reanimation: an update on nerve transfers in facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2019;27(4):231–236. https://doi.org/10.1097/MOO.0000000000000543
[5] Klebuc M, Shenaq SM. Donor nerve selection in facial reanimation surgery. Semin Plast Surg. 2004;18(1):53–60. https://doi.org/10.1055/s-2004-823124
[6] Terzis JK, Konofaos P. Nerve transfers in facial palsy. Facial Plast Surg. 2008;24(2):177–193. https://doi.org/10.1055/s-2008-1075833
[7] Yoshioka N. Differential reanimation of the midface and lower face using the masseteric and hypoglossal nerves for facial paralysis. Oper Neurosurg (Hagerstown). 2018;15(2):174–178. https://doi.org/10.1093/ons/opx217
[8] Owusu JA, Truong L, Kim JC. Facial nerve reconstruction with concurrent masseteric nerve transfer and cable grafting. JAMA Facial Plast Surg. 2016;18(5):335–339. https://doi.org/10.1001/jamafacial.2016.0345
[9] Yoshioka N, Tominaga S. Masseteric nerve transfer for short-term facial paralysis following skull base surgery. J Plast Reconstr Aesthet Surg. 2015;68(6):764–770. https://doi.org/10.1016/j.bjps.2015.02.031
[10] Lee YS, Ahn JH, Park HJ, et al. Dual coaptation of facial nerve using masseteric branch of trigeminal nerve for iatrogenic facial palsy: preliminary reports. Ann Otol Rhinol Laryngol. 2020;129(5):505–511. https://doi.org/10.1177/0003489419893722
[11] Biglioli F, Allevi F, Rabbiosi D, et al. Triple innervation for re-animation of recent facial paralysis. J Craniomaxillofac Surg. 2018;46(5):851–857. https://doi.org/10.1016/j.jcms.2018.02.014
[12] Chong LSH, Eviston TJ, Low TH, Hasmat S, Coulson SE, Clark JR. Validation of the clinician-graded electronic facial paralysis assessment. Plast Reconstr Surg. 2017;140:159–167. https://doi.org/10.1097/PRS.0000000000003447
[13] Terzis JK, Tzafetta K. The ‘babysitter’ procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg. 2009;123(3):865–876. https://doi.org/10.1097/PRS.0b013e31819ba4bb
[14] Mabvuure NT, Pinto-Lopes R, Bolton L, Tzafetta K. Lower lip depressor reanimation using anterior belly of digastric muscle transfer improves psychological wellbeing in facial palsy patients. Br J Oral Maxillofac Surg. 2022;60(3):299–307. https://doi.org/10.1016/j.bjoms.2021.07.025
[15] Bassilios Habre S, Googe BJ, Depew JB, Wallace RD, Konofaos P. Depressor reanimation after facial nerve paralysis. Ann Plast Surg. 2019 May;82(5):582–590. https://doi.org/10.1097/SAP.0000000000001616
[16] Vejbrink Kildal V, Tee R, Reissig L, Weninger WJ, Tzou CJ, Rodriguez-Lorenzo A. Selective ansa cervicalis nerve transfer to the marginal mandibular nerve for lower lip reanimation: An anatomical study in cadavers and a case report. Microsurgery. 2023; 43(2): 142–150. https://doi.org/10.1002/micr.30992
[17] Biglioli F, Guerra MB, Rabbiosi D, Ciardiello C, Allevi F. Comparison of results utilizing one-step and two-step triple innervation techniques. J Craniomaxillofac Surg. 2021;49(7):628–634. https://doi.org/10.1016/j.jcms.2021.03.006
[18] Admass BA, Ego BY, Tawye HY, Ahmed SA. Preoperative investigations for elective surgical patients in a resource limited setting: systematic review. Ann Med Surg (Lond). 2022 23;82:104777. https://doi.org/10.1016/j.amsu.2022.104777
[19] Telich-Tarriba JE, Velazquez E, Theurel-Cuevas A, et al. Upper extremity patterns of injury and management at a plastic and reconstructive surgery referral center in Mexico City. Ann Plast Surg. 2018;80(1):23–26. https://doi.org/10.1097/SAP.0000000000001182
[20] Varelas AN. Use of three or more concomitant nerve transfers for facial nerve reanimation. In: 14th international facial nerve symposium. Seoul, Korea, April 28–30, 2022. The Charles Bell Society, Soul, Korea.
[21] Dauwe PB, Hembd A, De La Concha-Blankenagel E, et al. The deep temporal nerve transfer: an anatomical feasibility study and implications for upper facial reanimation. Plast Reconstr Surg. 2016;138(3):498e–505e. https://doi.org/10.1097/PRS.0000000000002482