ORIGINAL RESEARCH ARTICLE
Treatment of orbital fractures – a critical analysis of ophthalmic outcomes and scenarios for re-intervention
Anna A. E. Perssona,b, Hanna M. Lifa,b, Alberto Falk-Delgadoc# and Daniel Nowinskia,b#
aDepartment of Plastic and Maxillofacial Surgery, Uppsala University Hospital, Uppsala, Sweden; bDepartment of Surgical Sciences, Uppsala University, Uppsala, Sweden; cDepartment of Plastic Surgery, Karolinska University Hospital, Stockholm, Sweden
Background: Malplaced implants in orbital reconstruction may lead to serious complications and necessitate re-intervention. The aim of this study was to describe outcomes, complications and scenarios of re-intervention in a historical case series of orbital fractures treated with free-hand orbital wall reconstruction. The main hypothesis was that early re-interventions are mainly because of malplaced implants in the posterior orbit.
Methods: Retrospective review of 90 patients with facial fractures involving the orbit, reconstructed with radiopaque orbital wall implants, from 2011 to 2016. Data were obtained from medical records and computed tomography images. Recorded parameters were fracture type, ocular injury, ocular motility, diplopia, eye position, complications and re-interventions. Secondary reconstructions because of enophthalmos were volumetrically evaluated.
Results: Early complications requiring re-intervention within 1 month were seen in 12 (13%) patients, where all except two were because of malplaced implants. The implant incongruence was without exception found in the posterior orbit. Late complications consisted of four (4%) cases of ectropion and five (5%) cases of entropion that needed corrective surgery. The majority of the patients with eye-lid complications had undergone repeated surgeries. Secondary orbital surgeries were performed in nine (10%) patients. Five of these patients had secondary reconstruction for enophthalmos and associated diplopia. None of these patients became completely free from either enophthalmos or diplopia after the secondary surgery.
Conclusion: Re-intervention after orbital reconstruction is mainly related to malplaced implants in the posterior orbit. Incomplete results in patients requiring secondary surgery for enophthalmos infer the importance of accurate restoration of the orbit at primary surgery.
Abstract presented at: Swedish surgery Week 2021 and SCAPLAS 2022.
KEYWORDS: Orbital fractures; orbital implant; outcome; complications; orbital volume; orbit; enophthalmos
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 1–7. DOI: https://doi.org/10.2340/jphs.v58.6580.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 9 January 2023; Accepted: 10 March 2023. Published: 2023-05-16.
CONTACT Anna Persson, MD anna.persson@akademiska.se Department of Plastic and Maxillofacial Surgery, Uppsala University Hospital, SE-751 85 Uppsala, Sweden
#Shared last authorship
Competing interests and funding: The authors have no conflicts of interest or financial disclosure to declare.
Introduction
Orbital wall reconstruction is an integral component in facial fracture repair, aiming to restore orbital wall anatomy, volume and function. The goal in orbital wall reconstruction is to prevent posttraumatic sequels, such as bulb malposition and diplopia [1–3].
While reconstruction of the orbit with orbital wall implants serves to prevent deformity and functional loss, the surgical treatment itself may lead to debilitating functional and aesthetic complications. Incorrect sizing, shaping and placement of implants may lead to inadequate restoration of orbital configuration and volume, as well as interference with extraocular muscles or cranial nerves [4]. The surgical access through the eyelid may also lead to complications related to eye-lid scaring [5]. Final outcomes are based on a comprehensive assessment of ocular and peri-orbital function and aesthetics. In patients with posttraumatic deformity or functional deficits, secondary surgery may be indicated [6].
Malplaced orbital wall implants visualised on postoperative computed tomography (CT) images may necessitate early re-intervention [7]. In the last two decades, there has been a strong development of computerised technologies for virtual surgical planning, navigation assisted surgery and intraoperative image analysis, all aiming to increase reconstructive precision, clinical outcomes and reducing the rate of re-operations [8]. However, there is a paucity of published reports with thorough analysis of the type, timing and causes of complications, re-intervention and secondary surgery after free-hand orbital fracture repair, without the use of aforementioned computerised technologies.
The purpose of this study was to describe outcomes of reconstructions with orbital implants, with emphasis on type of complications and re-interventions.
Material and methods
This treatment evaluation study of orbital floor fractures was approved by the committee of ethical vetting, Dnr 2016/405.
Subjects
Patients treated for facial fractures involving the orbit at the Department of Plastic and Maxillofacial Surgery, Uppsala University Hospital, from 2011 to 2016, were retrospectively studied. The inclusion criteria for the study were fractures reconstructed with orbital implant to orbital floor or orbital floor and medial wall combined. Patients lacking pre- and/or postoperative CT imaging and patients reconstructed with non-radiopaque implants or grafts were excluded from the study. Post-operative CT was habitually performed within 24 h of surgery to control the implant position. The routine clinical follow up was at 1 to 3 months after surgery. Patients with any residual symptoms were followed additionally for at least 6 to 12 months. Six of the patients (6%) were lost to clinical follow-up and therefore excluded from clinical outcome analysis. The data were collected from the charts in 2019, 3 years after the last performed surgery, which enabled a minimum of 3-year follow-up regarding complications.
Overall, 92 patients operated for orbital floor fractures with radiopaque implants were identified during the study period 2011–2016. These surgeries were performed by three plastic surgeons. In total, two patients were excluded from participation in the study. One patient was excluded because of a second time fracture of the same orbit, and another patient was excluded because of lack of post-operative CT. As three of the 90 remaining patients had bilateral orbital reconstructions, 93 orbits were left for analysis.
Data collection
The patients were identified through ICD-10 coding from the database for surgical planning. Patient data were collected from the electronic medical charts. The following data were included: age, sex, co-morbidity, preoperative assessments, perioperative data, re-interventions and clinical follow-up.
Eye position, ocular motility, stereovision and subjective visual impairment were clinically assessed by a plastic surgeon. All patients with ophthalmic symptoms were also assessed for associated ocular injuries by an ophthalmologist. In total, 59 (66%) patients were assessed by an ophthalmologist preoperatively. Based on all documented assessments, the findings were dichotomised as present or absent for the sake of this study. These data were missing in eight patients preoperatively, five of which because of intubation and sedation for severe brain injury.
Preoperative CT scans were examined, and orbital fractures were classified as isolated, zygomaticomaxillary (ZMC) or more complex. Isolated fractures were defined as fractures with an intact orbital rim or with a non-dislocated rim fracture not requiring reduction and osteosynthesis. In isolated orbital fractures, indications for surgery were CT-verified orbital fracture with ocular dysmotility because of mechanical extraocular muscle interference or orbital volume expansion where development of enophthalmos could be expected based on surgeons’ experience or was already present. More complex fractures were defined as orbital floor fractures with a dislocated orbital rim or ZMC fractures with additional facial fractures.
Based on the postoperative CT-scan images, the orbital implants maximum parasagittal depths from the infraorbital rim were measured in sagittal view. The orbital implants were also categorised according to the covering of the orbit as orbital floor or combined orbital floor-medial wall based on the CT images. The malplaced implants were evaluated and described qualitatively in all reoperated patients as too high or too low posteriorly and whether the implants were too long or too short for an optimal anatomical reconstruction. In addition, the CT images were examined to see whether any muscle interference could be seen.
Patients
Mean age at the time of injury was 46 years (range 16–82). The male to female ratio was 2:1. The most common cause of trauma was assault followed by falls and motor vehicle accidents (Table 1). Mean time from trauma to surgery was 11 days (range 1 to 58 days) and the median 10 days. In 73 patients (81%), surgical treatment was preformed within the first 14 days after trauma. Fifty (54%) of the orbital fractures were isolated, 13 (14%) were ZMC and 30 (32%) were classified as more complex (Table 2).
Surgical procedures
The preferred access to the inferior orbit was a pre-septal transconjunctival approach (89%), without lysis of the inferior limb of the lateral canthal attachment. Four operations were performed through a subciliary lower eye lid approach and one through a midlid incision. When more extensive exposure of the medial wall was required, a transconjunctival-transcaruncular approach was chosen in three cases. Twenty-one patients also had a bicoronal flap for access of the facial fractures.
The implants used were Medpore with titanium mesh (Stryker Leibinger) or solely titanium mesh (Synthes MatrixMIDFACE). All implants were screw fixated. In cases where the defect extended over both the orbital floor and lower half of the medial wall, a single combined implant was used to reconstruct the defect. More comprehensive defects were reconstructed with two implants. A single implant was used in 95% of the orbital reconstructions. Two implants were used in five cases (5%), all but one of them had complex fractures. In one patient, an extra porex sheet without titanium was placed above the implant to reduce orbital volume.
Total and partial orbital volume measurement
Total and partial orbital volumes were measured after the primary and secondary reconstruction on the unaffected and the affected side in the patients that underwent secondary reconstruction because of enophthalmos. For total orbital volume, the orbit was segmented in the semi-automatic segmentation software OrbSeg [9,10] and represented as a binary volume image. The OrbSeg software has been developed at the Centre for Image Analysis, Uppsala University, for image analysis in craniofacial research. For the purposes of the present study, a new data workflow was defined for measuring partial orbital volumes using the softwares OrbSeg 0.9.3 and ITK-SNAP 3.8.0, a software application used to segment structures in 3D medical images [10]. Firstly, in OrbSeg, five landmarks were placed onto the segmented orbit; three landmarks on specific voxels at the frontomaxillary suture (medial), the frontozygomatic suture (lateral) and at the most posterior centred voxel (posterior). Secondly, two landmarks were placed on the superior and inferior rim of the centred anterior orbit, their exact placement was calculated to define the symmetrical points between the medial and lateral landmarks. The anterior centre point was calculated between the two inferior and superior anterior landmarks. Then, four clipping planes were defined. Firstly, two clipping plane reference points were defined along the line between the centred anterior and posterior landmarks. This defined the anterior, middle and posterior thirds. The segmented orbit was divided into partial volumes based on the two anterior-posterior clipping planes and the medial-lateral and inferior-superior clipping planes. As a result, the orbital volume was divided into 12 parts. This enabled analysis of volumetric changes within the orbit from three perspectives: superior and inferior (divided into 1/2), anterior, mid and posterior (divided into 1/3) and lastly lateral and medial (divided into 1/2) (Figure 1).
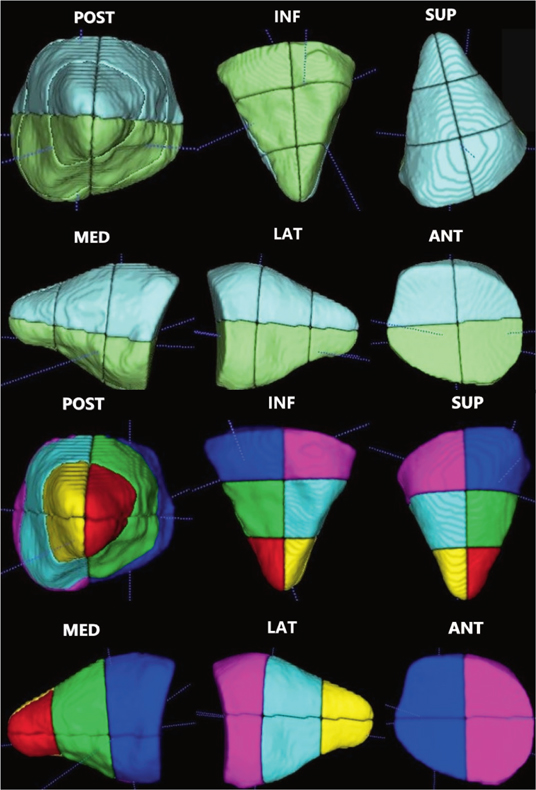
Figure 1. Partial orbital volumes.
Results
Associated eye injuries and ophthalmic outcomes
Associated eye injuries were found pre-operatively in 27 (46%) of the patients that were assessed by an ophthalmologist due to ophthalmic symptoms, which amounted to 30% of all patients. The most common eye injury was commotio retinae, followed by corneal injury, hyphema and optic neuropathy (Table 3). Two patients underwent enucleation because of bulb perforation because of the trauma.
For the study population as a whole, the postoperative status at last follow-up was found to be 5% subjective visual impairment, 5% ocular motility limitation, 10% enophthalmos and 26% diplopia (Table 4). Only five patients (6%) had a clinically significant enophthalmos at follow-up that required secondary reconstruction.
Early complications and re-intervention
A total of 12 patients (13%) suffered an early complication that required surgical re-intervention within 1 month. All but one of these 12 complications was implant related. There were 10 malplaced implants inside the orbit. Nine of these were detected at the post operative CT, and in one case restricted eye movement was found to be caused by a sharp edge of the implant interfering with the inferior rectus muscle, not detected initially on the post-operative CT. One implant related complication was a clinically palpable implant at the inferior orbital rim. The nonimplant-related reoperation was because of an abscess at a traumatic wound by the eyebrow, which had been used as an accessory access to the orbit at the primary surgery (Table 5). One patient underwent re-intervention twice within 1 month because of a malplaced and malshaped implant.
Qualitative analysis of the 10 malplaced implants inside the orbit found that the posterior edge of the orbital floor implant was elevated in five cases and depressed in three cases in relation to the posterior ledge of the orbital floor defect. Muscle interference was seen in four cases, and in all of these cases, the muscle interference was noted both clinically and on CT. There were two too short implants and two too long implants. The too short implants both resulted in a muscle trapping defect posteriorly. In the patient with two sequential re-interventions, the first implant was short and trapped the muscle as described above, while the second implant caused muscle interference because of relative elevation at its posterior edge (Table 6).
The re-interventions were performed at a mean of 6.3 and median 3.5 days after the primary surgery (range 1–22 days). Three (30%) of the 10 patients with malplaced implants requiring early re-intervention had persisting diplopia at long-term follow-up. None of the patients with early complications requiring re-intervention had subjective visual acuity disturbances or ocular motility restrictions at follow-up.
Secondary surgery
In total, nine patients (10%) required secondary surgery because of late complications. The time from trauma to secondary surgery was a median of 174 days (range 44 days to 715 days). Five of the nine patients had correction of enophthalmos, one of which had previously undergone an early re-intervention because of a malplaced implant. Four out of the five secondary enophthalmos corrections were performed in patients with complex midface fractures, one of which was a bilateral orbital fracture.
The four secondary surgeries not related to enophthalmos consisted of one patient who suffered diplopia because of posterior muscle entrapment caused by a too short implant, one patient with a palpable protruding implant at the inferior orbital rim and two patients with suspected implant related infections, where only one was verified by positive bacterial culture (Table 5).
Clinical improvement was seen in all five patients who underwent secondary surgery because of enophthalmos; however, none of them became completely free from either enophthalmos or diplopia. For one of these patients, some restriction of the ocular motility was found at the follow-up where scar tissue was believed to be the cause.
Orbital volume measurements were performed as described in Materials and Methods in order to better understand what caused the remaining enophthalmos and why the secondary reconstructions were insufficient. As the unaffected orbit was used as a reference, one patient with bilateral orbital fractures was excluded from this analysis.
Overall the volume difference in total was improved in the four patients analysed after the secondary surgery for enophthalmos. The median difference between the unaffected and affected orbit was 16% after the primary surgery and improved to 6% difference in volume after the secondary surgery. The improvements were made in the anterior and mid part of the orbit, while the posterior part in fact worsened to some degree in all the patients after the secondary surgery. For one patient, the secondary reconstruction was not made because of a volume issue but for an incomplete anatomical reconstruction, which was remedied with the secondary surgery (Table 7).
Late periorbital complications
Corrective eye lid surgery was performed in four (4%) patients that developed ectropion and in five (5%) patients with entropion. Six of these nine (67%) eyelid complications were found in the group of patients who had undergone repeated surgery (Table 2).
There was one patient who suffered a tear duct injury during a secondary surgery for enophthalmos. This specific patient had also undergone an early re-intervention because of malplacement of the implant during the primary operation.
Discussion
Orbital reconstruction can be challenging even to the most experienced surgeon. The orbit has a complex shape, it contains sensitive structures and limited operative view is offered. While surgery is intended to prevent aesthetic and functional post-traumatic sequels, it can also lead to iatrogenic injuries from the surgical exposure itself or from malplaced reconstruction implants [4].
Previous studies have shown a re-intervention rate for malplaced implants of 6.5–23% when peroperative CT or navigation is not used [7,11–14]. In our study 12 patients (13%) had an early complication requiring re-intervention within 1 month, most of these re-interventions were because of malpositioned implants. In all cases, the implant discrepancy was located in the posterior aspect of the orbital floor towards the orbital apex. Three patients were re-operated because of a too short implant allowing for posterior muscle entrapment, demonstrating the importance of striving towards complete orbital floor reconstruction to the posterior ledge of the defect. Indeed, the technical difficulties associated with deep orbital reconstruction are well recognised and related to limited visibility and the confined space of the orbital apex [14–16]. As all but one of the malplaced implants of the early complications were identified on the postoperative CT images, access to intraoperative CT would most probably have prevented these re-interventions [17,18]. Alternatively, improved precision in implant shaping and positioning could have been obtained by custom made implant and/or surgical navigation [14,19,20].
A 5% increase in orbital volume may be enough to cause clinically visible enophthalmos [21]. The reported incidence of secondary surgery for enophthalmos following orbital floor reconstruction ranges in the literature from 3 to 8% [4,6,22,23]. In general, the incidence of residual enophthalmos can be expected to increase in complex, high energy fractures [24]. Accordingly, four out of five patients operated secondarily for enophthalmos in this series had complex midface fractures. The cause of secondary enophthalmos is either inadequate reconstruction of the bony orbit and/or post-traumatic soft tissue changes such as fat atrophy and fibrosis [21,23,24]. Here, secondary surgery for enophthalmos was performed in 5% of the cases. What caused the enophthalmos in all cases cannot be determined in this study. However, in patient number three, partial orbital volume analysis indicates close to absolute anatomical reconstruction implying that soft tissue atrophy caused the persistent enophthalmos.
The transconjunctival approach is preferred by many surgeons as it does not leave a cutaneous scar and has been associated with a lower risk for ectropion and eye lid retractions. However, the transconjunctival access may be complicated by entropion [25], which is generally more challenging to correct than ectropion [5]. Here, the overall correction surgery rate for ectropion was 4 and 5% for entropion. The majority of patients (67%) requiring corrective eye lid surgery had gone through an orbital re-operation, emphasising the importance of avoiding repeated exposures through the lower eyelid.
Postoperative persisting diplopia has a reported incidence from 8 to 42% [2,6,26,27,28]. Here the incidence was 25% in total and 100% in those who underwent secondary surgery for enophthalmos. To prevent postoperative diplopia, early repair is advocated in cases where periorbital tissue may be trapped. In those patients who have persistent diplopia despite a seemingly adequate reconstruction, it is presumed that trauma to the muscle, fibrosis or nerve paresis is the reason for diplopia [29].
Visual impairment in general is poorly reported in orbital reconstruction studies. Vision loss has been reported between 0 and 0.4%, most often caused by intraaorbital haemorrhage. Here, subjective reduction of the visual acuity was found in 4% of all patients at follow-up. Vision loss was seen in two patients because of the trauma itself causing bulb perforation, requiring enucleation.
This study has strengths and limitations. Over these years, the surgery has had a stable surgical protocol in terms of indication, access approach and material, which enables analysis of inherent problems with free hand implant reconstruction. Patients were followed up as long as sequels and symptoms from the orbital trauma persisted and the number of patients lost to follow-up is very low related to other studies of facial trauma. Moreover, only one patient had to be excluded because of lack of post-operative CT and the series of 90 included patients compares well in size with previously published reports. All patients with any preoperative ophthalmic symptoms were assessed by an ophthalmologist, rendering validity to base-line data on ocular function. Advanced image analysis software enabled high-resolution measures of partial orbital volumes in a subset of patients with secondary enophthalmos, reflecting restoration of orbital anatomy. The major limitation of this study is that data on symptoms and clinical signs was collected retrospectively from non-standardised chart notes and not collected via prospectively designed study protocols. This precluded grading or more precise measures of symptoms and necessitated dichotomisation of data to avoid ambiguity. The study is descriptive in nature as a substantially larger population would be required for statistical analysis of potential predictors for outcomes.
Conclusion
This study demonstrates the challenges with precise anatomical reconstruction in the posterior orbit and confirms the importance of accurate restoration of the orbit at primary surgery to lower the morbidity. Secondary surgery was found to be even more demanding and re-operations demonstrated significant increased risk for late eye-lid complications.
Acknowledgement
We thank Johan Nysjö PhD, Daniel Isacson MD, PhD, Tia Liu MD and Daniel Saiepour MD, PhD for contributing to this study.
References
[1] Burnstine MA. Clinical recommendations for repair of orbital facial fractures. Curr Opin Ophthalmol. 2003;14(5):236–240. https://doi.org/10.1097/00055735-200310000-00002
[2] Biesman BS, Hornblass A, Lisman R, et al. Diplopia after surgical repair of orbital floor fractures. Ophthal Plast Reconstr Surg. 1996;12(1):9–16; discussion 17. https://doi.org/10.1097/00002341-199603000-00002
[3] Burnstine MA. Clinical recommendations for repair of isolated orbital floor fractures: an evidence-based analysis. Ophthalmology. 2002;109(7):1207–1210; discussion 1210–1211; quiz 1212–1213. https://doi.org/10.1016/s0161-6420(02)01057-6
[4] Senese O, Boutremans E, Gossiaux C, et al. Retrospective analysis of 79 patients with orbital floor fracture: outcomes and patient-reported satisfaction. Arch Craniofacial Surg. 2018;19(2):108–113. https://doi.org/10.7181/acfs.2018.01837
[5] Ridgway EB, Chen C, Colakoglu S, et al. The incidence of lower eyelid malposition after facial fracture repair: a retrospective study and meta-analysis comparing subtarsal, subciliary, and transconjunctival incisions. Plast Reconstr Surg. 2009;124(5):1578–1586. https://doi.org/10.1097/PRS.0b013e3181babb3d
[6] Hoşal BM, Beatty RL. Diplopia and enophthalmos after surgical repair of blowout fracture. Orbit Amst Neth. 2002;21(1):27–33. https://doi.org/10.1076/orbi.21.1.27.2598
[7] Schlittler F, Schmidli A, Wagner F, et al. What is the incidence of implant malpositioning and revision surgery after orbital repair? J Oral Maxillofac Surg. 2018;76(1):146–153. https://doi.org/10.1016/j.joms.2017.08.024
[8] Bly RA, Chang SH, Cudejkova M, et al. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15(2):113–120. https://doi.org/10.1001/jamafacial.2013.316
[9] Nysjö J. Interactive 3D image analysis for cranio-maxillofacial surgery planning and orthopedic applications. 2016. Acta Universitatis Upsaliensis, Uppsala.
[10] Khonsari RH, Hennocq Q, Nysjö J, et al. Defining critical ages for orbital shape changes after frontofacial advancement in crouzon syndrome. Plast Reconstr Surg. 2019;144(5):841e–852e. https://doi.org/10.1097/PRS.0000000000006162
[11] Wilde F, Lorenz K, Ebner AK, et al. Intraoperative imaging with a 3D C-arm system after zygomatico-orbital complex fracture reduction. J Oral Maxillofac Surg. 2013;71(5):894–910. https://doi.org/10.1016/j.joms.2012.10.031
[12] Nguyen E, Lockyer J, Erasmus J, et al. Improved outcomes of orbital reconstruction with intraoperative imaging and rapid prototyping. J Oral Maxillofac Surg. 2019;77(6):1211–1217. https://doi.org/10.1016/j.joms.2019.02.004
[13] Menon A, Karikal A, Shetty V. Does C-arm guidance improve reduction of zygomatic arch fractures? – a randomized controlled trial. J Oral Maxillofac Surg. 2018;76(11):2376–2386. https://doi.org/10.1016/j.joms.2018.05.026
[14] Nikunen M, Rajantie H, Marttila E, et al. Implant malposition and revision surgery in primary orbital fracture reconstructions. J Craniomaxillofac Surg. 2021;49(9):837–844. https://doi.org/10.1016/j.jcms.2021.04.008
[15] Evans BT, Webb AAC. Post-traumatic orbital reconstruction: anatomical landmarks and the concept of the deep orbit. Br J Oral Maxillofac Surg. 2007;45(3):183–189. https://doi.org/10.1016/j.bjoms.2006.08.003
[16] Saiepour D, Messo E, Hedlund AJO, et al. Radiologic and long-term clinical outcome from treatment of isolated medial orbital wall blowout fractures. J Craniofac Surg. 2012;23(5):1252–1255. https://doi.org/10.1097/SCS.0b013e31825e4e8e
[17] Anand L, Sealey C. Orbital fractures treated in Auckland from 2010–2015: review of patient outcomes. N Z Med J. 2017;130(1458):21–26.
[18] Gander T, Blumer M, Rostetter C, et al. Intraoperative 3-dimensional cone beam computed tomographic imaging during reconstruction of the zygoma and orbit. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(2):192–197. https://doi.org/10.1016/j.oooo.2018.04.008
[19] Zimmerer RM, Ellis E, Aniceto GS, et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J Craniomaxillofac Surg. 2016;44(9):1485–1497. https://doi.org/10.1016/j.jcms.2016.07.014
[20] Zavattero E, Ramieri G, Roccia F, et al. Comparison of the outcomes of complex orbital fracture repair with and without a surgical navigation system: a prospective cohort study with historical controls. Plast Reconstr Surg. 2017;139(4):957–965. https://doi.org/10.1097/PRS.0000000000003229
[21] Gart MS, Gosain AK. Evidence-based medicine: orbital floor fractures. Plast Reconstr Surg. 2014;134(6):1345–1355. https://doi.org/10.1097/PRS.0000000000000719
[22] Nowinski D, Messo E, Hedlund A. Treatment of orbital fractures: evaluation of surgical techniques and materials for reconstruction. J Craniofac Surg. 2010;21(4):1033–1037. https://doi.org/10.1097/SCS.0b013e3181e4345d
[23] Zimmerer RM, Gellrich NC, von Bülow S, et al. Is there more to the clinical outcome in posttraumatic reconstruction of the inferior and medial orbital walls than accuracy of implant placement and implant surface contouring? A prospective multicenter study to identify predictors of clinical outcome. J Craniomaxillofac Surg. 2018;46(4):578–587. https://doi.org/10.1016/j.jcms.2018.01.007
[24] Chen CT, Huang F, Chen YR. Management of posttraumatic enophthalmos. Chang Gung Med J. 2006;29(3):251–261.
[25] Pausch NC, Sirintawat N, Wagner R, et al. Lower eyelid complications associated with transconjunctival versus subciliary approaches to orbital floor fractures. Oral Maxillofac Surg. 2016;20(1):51–55. https://doi.org/10.1007/s10006-015-0526-1
[26] Chi MJ, Ku M, Shin KH, et al. An analysis of 733 surgically treated blowout fractures. Ophthalmologica. 2010;224(3):167–175. https://doi.org/10.1159/000238932
[27] Greenwald HS, Keeney AH, Shannon GM. A review of 128 patients with orbital fractures. Am J Ophthalmol. 1974;78(4):655–664. https://doi.org/10.1016/s0002-9394(14)76304-4
[28] Brucoli M, Arcuri F, Cavenaghi R, et al. Analysis of complications after surgical repair of orbital fractures. J Craniofac Surg. 2011;22(4):1387–1390. https://doi.org/10.1097/SCS.0b013e31821cc317
[29] Cole P, Kaufman Y, Hollier L. Principles of facial trauma: orbital fracture management. J Craniofac Surg. 2009;20(1):101–104. https://doi.org/10.1097/SCS.0b013e318190e1b6