ORIGINAL RESEARCH ARTICLE
Diagnostical accuracy of hyperspectral imaging after free flap surgery
Torsten Schulza, Rima Nuwayhida, Khosrow Siamak Houschyarb, Stefan Langera and Lukas Kohlera,c
aDepartment of Orthopedic, Trauma and Plastic Surgery, University Hospital Leipzig, Leipzig, Germany; bHautzentrum Köln, Cologne, Germany; cDivision of Hand-, Plastic- and Aesthetic Surgery, University Hospital Munich, Munich, Germany
ABSTRACT
Microsurgical free-tissue transfer has been a safe option for tissue reconstruction. This study aimed to analyze the diagnostic accuracy of hyperspectral imaging (HSI) after free-tissue transfer surgery.
From January 2017 to October 2019, 42 consecutive free-flap surgeries were performed, and their outcomes were analyzed via HSI. Clinical examination of free-flap perfusion was initially performed. Clinical examination findings were subsequently compared with those of HSI. Potential venous congestion with subsequent necrosis was defined as a tissue hemoglobin index of ≥53%. Student’s t-test was used to compare the results of the analysis. The evaluation of sensitivity and specificity for flap failure detection was time dependent using the Fisher’s exact test. A p-value of ≤0.05 was considered statistically significant.
Microsurgical tissue transfer success rate was 84%. Seven patients presented with venous congestion that caused total flap necrosis. Overall, 124 assessments were made. HSI accurately identified 12 out of 19 pathological images: four as false positive and seven as false negative. The sensitivity and specificity of HSI were 57 and 94%, respectively, compared to those of clinical examination that were 28 and 100%, respectively, within 24 h following tissue transfer. The sensitivity and specificity of HSI were 63 and 96%, respectively, compared to those of clinical examination that were 63 and 100%, respectively, within the first 72 h.
A tissue hemoglobin index of ≥53% could predict venous congestion after free-flap surgery. HSI demonstrated higher sensitivity than clinical examination within the first 24 h; however, it was not superior compared to clinical findings within 72 h.
KEYWORDS: Hyperspectral imaging; free flap; malperfusion; venous congestion
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 48–55. DOI: https://doi.org/10.2340/jphs.v58.7140.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 25 February 2023; Accepted: 25 May 2023; Published: 24 August 2023.
CONTACT Torsten Schulz, MD torsten.schulz1988@gmail.com Department of Orthopedic, Trauma and Plastic Surgery, University Hospital Leipzig, Liebigstraße 20, 04103 Leipzig
Competing interests and funding: The authors have no conflicts of interests to declare.
Introduction
Complex tissue defect reconstruction via free-flap surgery is safe and reliable [1]. Owing to technical progress and microsurgical expertise, the success rate has continually increased to approximately 95% [2]. Despite these advances, ischemia-related complications can still occur after pedicelled or free-tissue transfer, resulting in partial or complete flap loss. Therefore, microsurgical management aims to continually reduce ischemia-related morbidity and facilitate safe reconstruction [3]. Generally, reconstructive surgery clinics rely on the clinical assessment of visual perfusion control (that is, a recapilarization time of 3 s within the first 72 h) [4]. Different technical devices have been introduced; however, they have several requirements (such as wide availability, neutral cost and personal resources, easy data interpretation, independence from the surgical method, and reliability) [4]. Hyperspectral imaging (HSI) was first used in human medicine approximately 10 years ago, and subsequently, it has been applied in several scientific areas [5, 6]. To date, five different studies have been published on the clinical use of HSI for flap monitoring in tissue reconstructive surgery [7]. Recent studies suggested a decreased oxygenation as an indication of flap malperfusion. However, different thresholds were postulated [8, 9]. In head and neck surgery, superficial or deep tissue oxygenation thresholds were hypothesized to be the optimal parameters for operative revisions in different kinds of impaired flap perfusion (arterial occlusion or venous congestion). Other parameters like hemoglobin or water concentrations in the tissue were not mentioned [9]. Furthermore, distributions between the different kinds of malperfusion were not considered. Our own working group prospectively examined a smaller group of patients with respect to normal and pathological perfusion after microsurgical defect repair. Among other things, it was concluded that a tissue hemoglobin index (THI) of over 53% indicates venous congestion [10]. Therefore, this study aimed to assess the perfusion of free flaps postoperatively, compare our internal analysis software algorithm with the clinical findings of surgeons, and describe the accuracy of HSI using a contingency table. The aim was to proof our hypothesis based on a former study that a THI detects a venous malperfusion with an adequate diagnostical accuracy. Therefore, we hypothesized that a THI of ≥53% can accurately indicate venous congestion.
Material and methods
Patients
In this retrospective, non-randomized study, patients who required free flap reconstruction of the trunk and extremities were included. This study was performed using an internal HSI database. From January 2017 to October 2019, datasets of 31 men and nine women (median age: 55 ± 15.7 [26–92] years) who underwent free-flap surgery were recorded. Only those with free flaps were included, whereas those with buried flaps and invalid datasets were excluded. Clinical and instrumental examinations were performed by physicians. Defect etiology, comorbidities, and flap recipient site data were recorded.
The primary outcomes were divided into two: regular flap healing and postoperative flap necrosis (Figure 1(a)). Microvascular free-flap raising was performed according to standard procedures. After microsurgical anastomoses, tissue perfusion was initially assessed by standard clinical examinations (capillary blanching and refill, skin color, and dermal bleeding). Thereafter, tissue perfusion was evaluated by HSI. Hypo- or malperfused flaps were marked after HSI assessment and compared with clinical findings. Based on the measurements’ temporal nature, three groups were classified as follows: measurements ≤24 h (t1), measurements between 24 and 48 h (t2), and measurements between 48 and 72 h (t3). Arterial occlusion was defined as a sudden pale color, prolonged or absent capillary refill time, and no bleeding, whereas venous congestion was defined as a blue color, immediate capillary refill, deep red bleeding on puncture, and subsequent flap necrosis. For this purpose, a marker was placed over the central parts of the free flaps using the manufacturer’s software, and the corresponding values were recorded. Data on clinical assessment and HSI findings were entered into our departmental database. Due to the study’s retrospective nature, the final decision to reexplore was made based on clinical evaluation.
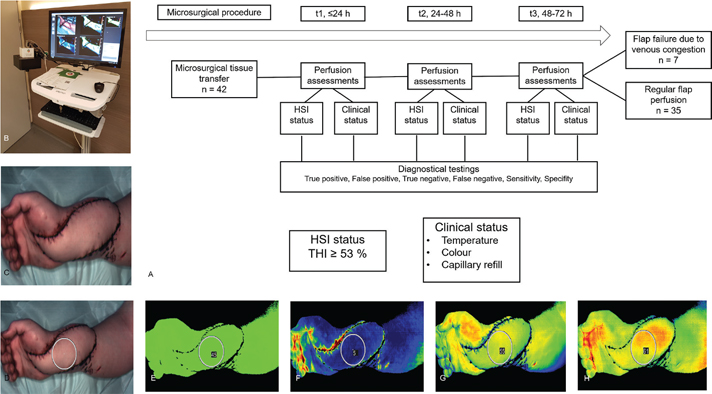
Figure 1. a–h. Study protocol and case number 39. ALT flap for a right hand defect covering. a. Study protocol. b. The TIVITA Tissue hyperspectral camera system. c. The underlying flap was illuminated by a white light source. d. An image was captured, and a marker was placed over the region of interest. e. Average oxygenation of the tissue (StO2) with a marker showing oxygen concentrations in the viable flap area. f. Tissue hemoglobin index (THI). g. Near-infrared (NIR) perfusion index. h. Tissue water index (TWI).
HSI and technical details
Postoperative HSI was performed using TIVITA© Tissue (Figure 1(b–h)) via Diaspective Vision GmbH (Strandstrasse 15, D-182333 Am Salzhaff, Germany). The system comprises a lighting unit of two 120-W halogen lamps, an imaging spectrometer, and a complementary metal-oxide-semiconductor camera of 640 × 480 pixels resolution for image recording. HSI can measure tissue perfusion discontinuously and is noninvasive, recordable, and easy to use; a single measurement lasts for 5 s. The distance between the camera and tissue was set at 50 cm. A standardized area of 30 × 30 cm can be assessed using HSI. Light reduction (such as sunlight and room lighting) was recommended for optimal data quality. The underlying principle of HSI was based on spectroscopic remission measurements in the visible and near-infrared spectral range of 500–995 nm. The target was illuminated, and the altered light reflected from the tissue was displayed. Hemoglobin is the predominant optical component of the tissues, and oxygenated and reduced hemoglobin level varies between vital and ischemic tissues. Visible light tissue penetration depth of approximately 0.5 mm and a near-infrared spectral range of 3–5 mm influence upper tissue layer (microcirculation) and blood flow parameters at a deeper depth [6]. The four parameters measured using the software were the hemoglobin oxygenation at the superficial skin (StO2), hemoglobin concentration at the superficial level (THI), near-infrared spectroscopy (NIR), and the tissue water index (TWI) [6, 11]. A THI of ≥53%, NIR of ≤25%, and TWI of ≤43% indicated a locally impaired perfusion, and a THI of ≥53% in the center of the flap indicated venous congestion [10]. Images were assessed using TIVITA Suite© (Diaspective Vision, Germany). The study was conducted according to principles of the Declaration of Helsinki (updated in 2008) and approved by the ethics committee of the Ärztekammer Sachsen-Anhalt, Germany (Approval 35-17).
Free flap skin paddles were examined. First, the flap was inspected clinically for malperfusion signs. A marker of maximum size was placed in the center of the flap without touching the soft tissue or suture material. Venous congestion with subsequent necrosis was defined as a THI of ≥53%. After image acquisition, the four parameters of the marked areas were calculated using the analysis software provided by the manufacturer.
Statistical analysis
All statistical analyses were performed using Xlstat© (version 2020.3.1, Addinsoft, New York, USA). Means with standard deviations or numbers with percentages were used for descriptive statistics. To compare two sets of data, a student’s t-test was used. To assess the diagnostic reliability of postoperative clinical findings or HSI assessments, sensitivity and specificity were calculated and presented in a contingency table. A p-value of <0.05 was considered statistically significant.
Results
Overall, data on 42 free-flap surgeries were analyzed. The mean age was 56.7 ± 15.4 years. Table 1 summarizes the characteristics of patients and clinical data. The free flaps included 25 anterolateral thigh (ALT), 10 latissimus dorsi muscle (LDM), five deep inferior epigastric perforator (DIEP) flaps, one parascapular (PSC), and one rectus abdominis flap. The reason for reconstruction included traumatic injuries (n = 32, 76.2%), wound infections (n = 6, 14.2%), oncologic defects (n = 3, 7.2%), and peripheral occlusive diseases (n = 1, 2.4%). The lower extremity was the most common recipient site (n = 30, 71.4%), followed by the upper extremity (n = 8, 19.0%), chest (n = 2, 4.8%), head (n = 1, 2.4%), and abdomen (n = 1, 2.4%). A total of 35 patients underwent successful reconstruction. Hence, the overall flap success rate was 84%. However, 16% of the reconstructive cases resulted in flap failure, requiring further surgical intervention. The mean length of hospital stay was 34 ± 26 (range: 12–124) days. The hyperspectral datasets of each patient were recorded based on perfusion status and time. Arterial occlusion malperfusion was not observed.
HSI data
The corresponding HSI data of each patient were recorded. In total, 124 records were prepared, and 101 measurements were made on the viable flap areas. Moreover, 19 free flap datasets with venous congestion were analyzed. The average StO2 was 44.5 ± 15.2% [3–80], NIR, 41.0 ± 11% [7–72], THI, 27.2 ± 20.1% [0–93], and TWI, 50.5 ± 8.4% [34–74] for viable flaps on the first day (Figure 2(a–d)). Free flaps with impaired perfusion in the form of venous congestion were characterized by decreased StO2 (35.5%), NIR (26.3%), and TWI (47,3%) and increased THI (approximately 64.3%) after 3 days. Student’s t-test was used to compare each parameter between the groups. No significant differences were observed for StO2 between viable free flaps and flaps with malperfusion caused by venous congestion at each time point. However, regarding NIR, THI, and TWI, statistically significant differences were observed.
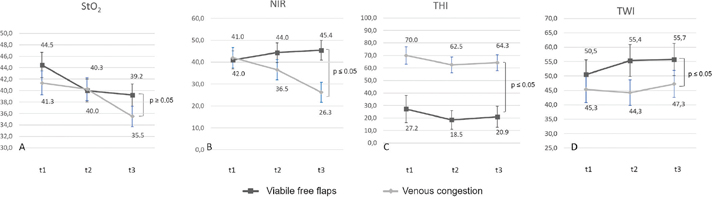
Figure 2. StO2, THI, NIR, and TWI based on different perfusion status. a. Decreasing StO2 values of both groups. b. Reduced NIR indicating venous congestion, requiring major revisions. c. Increasing THI between venous congestion and viable free flaps. d. Increasing TWI between viable free flaps and venous congestion.
Subgroup t1: Within the first 24 h, case 31 developed venous congestion malperfusion during clinical and instrumental assessments. Operative revision was required. Intraoperatively, the venous anastomoses were thrombosed; therefore, the flap was directly removed. Further measurements could not be performed. Cases 20, 2, and 10 demonstrated a regular clinical appearance and were not revised. However, the HSI camera indicated early venous congestion. The clinical situation was rated as unremarkable. However, venous congestion further deteriorated in period t3 such that an operative revision was finally initiated, and the suspicion was confirmed intraoperatively as a thrombosed vein. The cases were rated as true positive.
Cases 9 and 33 had clinically viable flaps. However, there was a discrepancy between clinical findings, indicating overall good flap perfusion, and HSI, suggesting venous congestion. Postoperative healing was uneventful, and a revision did not occur. The cases were marked as false positives. The HSI measurements showed a sensitivity of 57%, a specificity of 94%, a positive predictive value of 67%, and a negative predictive value of 91%. The clinical examination results, which can be viewed from the files, showed a sensitivity of 28%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 87% (Figure 3(a)).
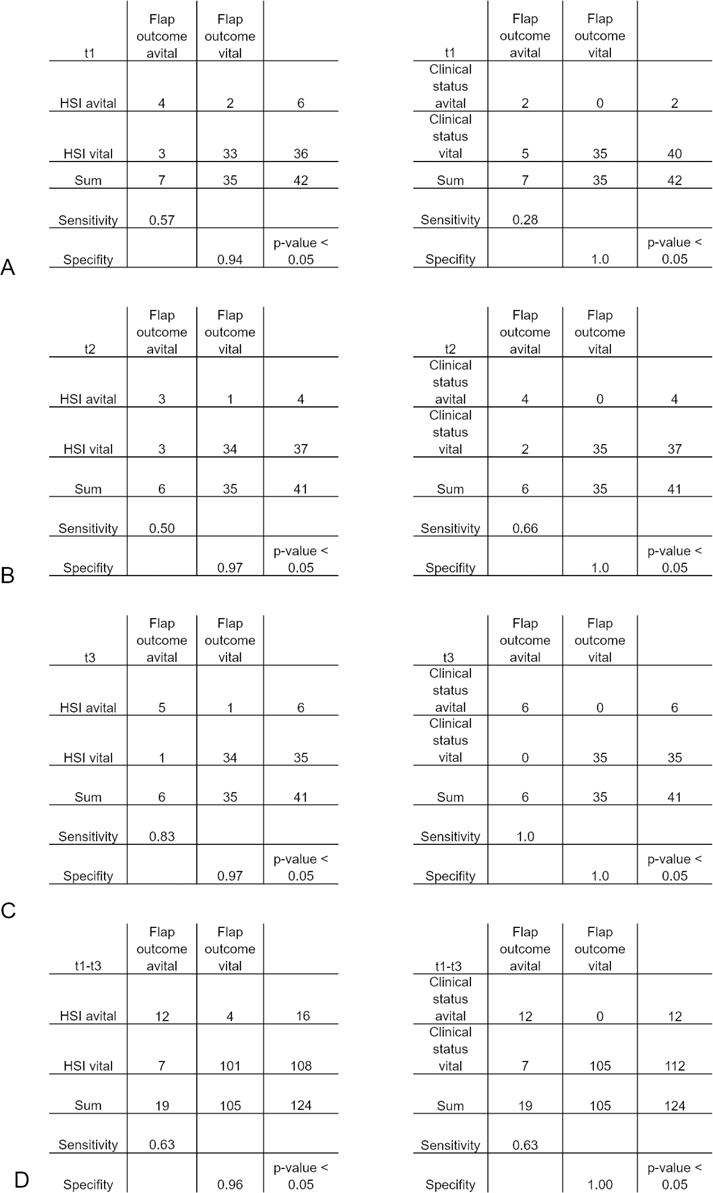
Figure 3. A direct comparison between the diagnostical accuracy of HSI and clinical examination using a contingency table. a. Comparison between clinical examination and HSI for detecting flap malperfusion within 24 h. b. Comparison between clinical examination and HSI for detecting flap malperfusion between 24 and 48 h. c. Comparison between clinical examination and HSI for detecting flap malperfusion between 48 and 72 h. d. Summary and comparison of both monitoring modalities characterized by contingency tables within the first 72 h.
Subgroup t2: Between 24 and 48 h, cases 2 and 10 presented with further progressive venous congestion during clinical and instrumental assessments. In case 31, measurements could no longer be taken. Every case was marked as true positive. Case 33 was indicated for major revision based on HSI findings. However, there were no clinical data regarding the signs of malperfusion or necrosis. Therefore, case 33 was marked as false positive. In contrast, case 9 was no longer indicated for revision based on HSI findings after 24 h and was marked as true positive. In the second subgroup, HSI measurements showed a sensitivity of 50%, a specificity of 97%, a positive predictive value of 75%, and a negative predictive value of 91%. The clinical investigations showed a comparable sensitivity of 66%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94% (Figure 3(b)).
Subgroup t3: Between 48 and 72 h after free-flap surgery, cases 4 and 26 had prolonged recapilarization caused by venous congestion. Clinical and hyperspectral data indicated and confirmed venous congestion caused by venous thrombosis between 48 and 72 h postoperation. The decision to discard the flap in both cases was validated with multiple thromboses. Both flaps required surgical debridement and either flap advancement or additional tissue reconstruction via a second free flap. Cases 2, 10, 20, and 41 presented with progressive necrosis of the whole flap caused by secondary venous thrombosis. HSI measurements demonstrated a sensitivity of 83%, a specificity of 97%, a positive predictive value of 83%, and a negative predictive value of 97% in the last subgroup, whereas the clinical examination of free flaps demonstrated a sensitivity of 100%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 100% (Figure 3(c)).
In summary, 124 records were made during the study period. Of these 124 measurements, HSI showed poor flap perfusion in 16 records within 72 h. Seven flaps were lost during the study period. HSI accurately identified venous congestion in 12 of 19 assessments of free flaps. Four assessments were marked as false positive (cases 9 and 33), and seven assessments were marked as false negative (Figure 3). Based on these data, the sensitivity and specificity of HSI were 63 and 96%, respectively (p < 0.001), within the first 72 h. Regarding the clinical examinations, 124 examination results were evaluated within the first 72 h. Of these, 12 examinations were rated as true positive, zero as false positive, seven as false negative, and 105 as true negative. This resulted in a sensitivity of 63% and a specificity of 100% (Figure 3(d)).
Discussion
A previous study showed that the microvascular free-tissue transfer success rate was >90% [12]. Despite the good outcomes of head and neck surgery, the outcomes of older individuals and those with obesity and atherosclerosis are challenging to manage. If a malperfusion of a free flap occurs, prompt reintervention improves flap salvage [13].
The technically noninvasive monitoring methods include NIR [14], laser Doppler flowmetry/tissue spectrophotometry [15], and HSI [10] (Table 2). NIR has been used clinically since 1995 [16] with several advantages. In a retrospective trial of 900 microsurgical breast reconstructions, NIR significantly decreased the flap loss rate and improved the flap salvage rate [17]. In another retrospective study of 1050 patients who underwent microsurgical tissue reconstruction, the flap salvage rate increased to 96.6%, the number of complete flap losses decreased, and the rate of reintervention decreased over time [18]. In a systematic review, the overall flap success rate was 99.5%, and the flap salvage rate was 91.1% [14]. However, compared with laser flowmetry, NIR cannot predict wound complications, including fat necrosis [19], or provide information on blood flow [20].
| Method | HSI [6, 21] | NIR [14, 22] | Laser Doppler flowmetry/tissue spectrophotometry [15] |
| Device/company | Tivita Tissue®/Diaspective Vision, Germany | Tissue Oximeter®/T.Ox, the USA | O2C®LEA Medizintechnik, Germany |
| Range | 500–1000 nm | 690–850 nm | 830 nm/500–800 nm |
| Modality | Noninvasive | Noninvasive | Noninvasive |
| Assessment frequency | Discontinuous | Every 2–4 s | Continuous |
| Monitoring modality | Imaging (30 × 30 cm) | No imaging (probe) | No imaging (probe) |
Laser Doppler flowmetry, similar to the O2C device, can determine blood flow (flow), velocity, hemoglobin oxygenation, and relative hemoglobin concentrations [15]. Furthermore, the O2C device can provide data on intracapillary hemoglobin oxygenation in correlation with intracapillary hemoglobin saturation and the local blood flow rate [15]. Vessel occlusion was detected in five of five patients who underwent free-flap breast reconstruction. Thus, O2C may improve flap survival rates at an early stage [15]. The disadvantage of the O2C device is its cost and cautious placement of sensor as shearing motions and excessive pressure can lead to false measurements [15]. HSI and NIR are both optical devices for measuring tissue perfusion in different electromagnetic ranges. In contrast to O2C or NIR, HSI can facilitate a contact-free, noninvasive imaging analysis. In a recent systematic review, the most frequently reported limitations of clinical monitoring were the need for expert interpretation (25% of related papers), unsuitability of buried flaps (21%), and a lack of quantitative/objective values (19%) [23]. To provide sufficient quantitative data and reduce the need for expert interpretation, different diagnostical studies were published to establish quantitative thresholds for HSI in microsurgical tissue reconstruction in recent years [8–10, 24, 25]. The studies were prospective in nature, had small sample sizes, and had a reexploration rate based on clinical assessments. Furthermore, the studies postulated that malperfusion goes hand in hand with reduced oxygen saturation in form of a decreased StO2 and NIR. Due to the low number of cases and different kinds of flap surgeries, different thresholds were postulated (Table 3). However, a distinction between arterial occlusion and venous congestion was not made in every trial. Therefore, the role of other parameters, such as the THI or TWI, remains unclear.
| Author | Thiem et al. [9] | Thiem et al. [24] | Kohler et al. [8] | Schulz et al. [10] |
| year | 2021 | 2020 | 2021 | 2021 |
| design | prospective | prospective | prospective | prospective |
| cases | 63 | 30 | 22 | 19 |
| distinction between arterial occlusion and venous congestion | none | yes | none | yes |
| thresholds | StO2 ≤ 32% StO2∆reference > −38% NRI ≤ 32.9 NRI∆reference ≥ −13.4% |
StO2 < 40% NIR < 25% THI < 40% |
NIR ≤ 40 | THI ≥ 53 % NIR ≤ 25 % TWI ≤ 43% StO2 ≤ 22% |
For the first time, our study has attempted to introduce the role of THI into clinical focus and derive a therapeutic outcome from it. In our study, HSI predicted poor tissue perfusion in all seven patients with total flap necrosis and confirmed adequate tissue perfusion in 105 of 125 measurements (specificity: nearly 84%). Only four measurements were documented as false negative. In the case of venous congestion, a decrease in StO2 and NIR was observed in subgroups t1, t2, and t3. However, statistical significance was observed only for NIR, which is in contrast to the previous publications (Table 3). However, THI was statistically significant in subgroup A in differentiating between vital and non-vital flaps as a dichotomous target variable. This diagnostic study showed that in the case of venous congestion of free flaps, THI plays an essential role as the StO2 or NIR. A THI ≥53% seems to indicate venous stasis. A simultaneous decrease in NIR and StO2 suggests venous congestion or arterial occlusion [10]. The false positive results in cases 9 and 33 may be due to postoperative reactive hyperemia of both flaps. In both cases, the intraoperative anastomosis of a second vein was not considered. As a result, the venous flow may have increased compared with free flaps with two anastomosed veins, and a higher proportion of hemoglobin may have accumulated. The false negative assessments in cases 4, 26, and 41 may be because the assessments were performed between 48 and 72 h. Previously, there had been a prolonged malperfusion of these flaps that could explain the final drop in THI as an expression of necrosis of the superficial skin tissue that had already begun.
The major role of HSI was the early pre-clinical identification of flap compromises. Thiem et al. in a previous publication stated that HSI defects perfusion compromises significantly earlier than clinical monitoring based on decreased StO2 or NIR [9]. The same would have to be investigated regarding THI. In cases 2 and 10, HSI could report an indication for a revision within the first 24 h (t1). However, clinical decision was taken on the following days. In this case, based on the HSI data, an earlier operational revision could have been initiated. Within the first 24 h, HSI showed a higher sensitivity in detecting venous congestion in comparison to clinical examination. However, in the other periods, no diagnostic superiority of HSI over the clinical examination was observed. Nevertheless, no higher sensitivity or specificity was demonstrated within the first 72 h following a direct comparison between clinical examination and the use of HSI. A possible explanation may be the use of a threshold ≥53% for this analysis. This threshold was validated in a smaller patient cohort [10]. Another possible explanation would be the fact that the investigators were physicians with many years of clinical practice and are particularly experienced in assessing free flaps. The comparison between the use of HSI and the clinical examination of nurses may be different, among other things. However, due to the study’s retrospective nature, this cannot be directly implemented, and further prospective studies are necessary.
To ensure clarity and better control of massive amount of data, artificial intelligence could be a suitable alternative for predicting malperfusion. A large number of studies have been published in which diagnostic statements were made based on the data generated by HSI. For example, different types of mouth tissue could be classified by their HS-signature using a deep learning approach [26]. In addition, an algorithm for brain cancer classification [27] or the differentiation between skin complications in diabetes mellitus and healthy volunteers using HSI was demonstrated [28]. Further investigation of flap perfusion by artificial intelligence may be required. The lack of quantitative values and necessary interpretation for noninvasive flap monitoring devices could thus be remedied. However, the possible disadvantages of artificial intelligence include the large amounts of data that are required and the corresponding cost of programming.
A disadvantage of HSI compared to other diagnostic tools is the relatively cumbersome handling, the discontinuous measurement process, the susceptibility to interference due to external light sources, and the fact that only tissue with an intact skin layer can be measured. Flaps without a skin island cannot be measured.
This study had several limitations. First, it was retrospective in nature. The fact that the THI ≥ 53% was a retrospectively fitted threshold could lead to massive overfitting in future research. The study would be more relevant if new patients were included in a prospective study, and the same threshold is used to differentiate between physiological perfusion and stasis. Moreover, because of the relatively small sample size, partial flap necrosis information was not examined, and there were missing data about flaps with arterial occlusion. Second, the measurements did not affect the usual clinical care; therefore, the flap salvage rate was not reproducible. The diagnostic value of postoperative assessment is, at least, defined by the flap salvage rate [17]. Fourth, the occurrence of late-onset complications, including wound edge necrosis, was not assessed [19].
Conclusions
HSI can detect impaired flap perfusion caused by venous stasis, which is defined as a THI of ≥53%. HSI demonstrated a higher sensitivity compared to clinical examination within the first 24 h. THI as a marker for the detection of malperfusion requires further assessment.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
The ethics committee of the Ärztekammer Sachsen-Anhalt, Germany, has approved the feasibility of the study prior to study initiation (Approval 35-17). The study and all methods were conducted following the principles of the Declaration of Helsinki (updated in 2008). Moreover, all patients signed a preoperative informed consent form and agreed to participate in the accompanying scientific research.
This work was supported by Diaspective vision (provision of the cameras used during the measurements).
References
[1] Ludolph I, Lehnhardt M, Arkudas A, et al. Plastic reconstructive microsurgery in the elderly patient – consensus statement of the German Speaking Working Group for Microsurgery of the Peripheral Nerves and Vessels. Handchir Mikrochir Plast Chir. 2018;50(2):118–125. https://doi.org/10.1055/s-0043-115730
[2] Zhang Y, Gazyakan E, Bigdeli AK, et al. Soft tissue free flap for reconstruction of upper extremities: a meta-analysis on outcome and safety. Microsurgery. 2019;39(5):463–475. https://doi.org/10.1002/micr.30460
[3] Schmauss D, Beier JP, Eisenhardt SU, et al. The “safe” flap – preoperative perforator-mapping and intraoperative perfusion assessment to reduce flap-associated morbidity – consensus statement of the German Speaking Working Group for Microsurgery of the Peripheral Nerves and Vessels. Handchir Mikrochir Plast Chir. 2019;51(6):410–417. https://doi.org/10.1055/a-0987-0118
[4] Wallner C, Kolbenschlag J, Daigeler A, et al. Perioperative management in microsurgery – consensus statement of the German-speaking Working Group for Microsurgery of the Peripheral Nerves and Vessels. Handchir Mikrochir Plast Chir. 2020;52(4):310–315. https://doi.org/10.1055/a-1205-1309
[5] Yudovsky D, Nouvong A, Pilon L. Hyperspectral imaging in diabetic foot wound care. J Diabetes Sci Technol. 2010;4(5):1099–1113. https://doi.org/10.1177/193229681000400508
[6] Holmer A, Tetschke F, Marotz J, et al. Oxygenation and perfusion monitoring with a hyperspectral camera system for chemical based tissue analysis of skin and organs. Physiol Meas. 2016;37(11):2064–2078. https://doi.org/10.1088/0967-3334/37/11/2064
[7] Lindelauf A, Saelmans A, van Kuijk S, et al. Near-infrared spectroscopy (NIRS) versus hyperspectral imaging (HSI) to detect flap failure in reconstructive surgery: a systematic review. Life. 2022;12(1):65. https://doi.org/10.3390/life12010065
[8] Kohler L, Köhler H, Kohler S, et al. Hyperspectral Imaging (HSI) as a new diagnostic tool in free flap monitoring for soft tissue reconstruction: a proof of concept study. BMC Surg. 2021;21(1):222. https://doi.org/10.1186/s12893-021-01232-0
[9] Thiem D, Romer P, Blatt S, et al. New approach to the old challenge of free flap monitoring-hyperspectral imaging outperforms clinical assessment by earlier detection of perfusion failure. J Pers Med. 2021;11(11):1101. https://doi.org/10.3390/jpm11111101
[10] Schulz T, Leuschner S, Siemers F, et al. Assessing flap perfusion after free tissue transfer using hyperspectral imaging (HSI). Eur J Plast Surg. 2021;44(4):1–10. https://doi.org/10.1007/s00238-021-01784-7
[11] Duann JR, Jan CI, Ou-Yang M, et al. Separating spectral mixtures in hyperspectral image data using independent component analysis: validation with oral cancer tissue sections. J Biomed Opt. 2013;18(12):126005. https://doi.org/10.1117/1.JBO.18.12.126005
[12] Kubo T, Yano K, Hosokawa K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery. 2002;22(8):391–395. https://doi.org/10.1002/micr.10059
[13] Smit JM, Acosta R, Zeebregts CJ, et al. Early reintervention of compromised free flaps improves success rate. Microsurgery. 2007;27(7):612–616. https://doi.org/10.1002/micr.20412
[14] Kagaya Y, Miyamoto S. A systematic review of near-infrared spectroscopy in flap monitoring: current basic and clinical evidence and prospects. J Plast Reconstr Aesthet Surg. 2018;71(2):246–257. https://doi.org/10.1016/j.bjps.2017.10.020
[15] Rothenberger J, Amr A, Schaller HE, et al. Evaluation of a non-invasive monitoring method for free flap breast reconstruction using laser doppler flowmetrie and tissue spectrophotometry. Microsurgery. 2013;33(5):350–357. https://doi.org/10.1002/micr.22096
[16] Irwin MS, Thorniley MS, Doré CJ, et al. Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg. 1995;48(1):14–22. https://doi.org/10.1016/0007-1226(95)90024-1
[17] Lin SJ, Nguyen MD, Chen C, et al. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast Reconstr Surg. 2011;127(3):1080–1085. https://doi.org/10.1097/PRS.0b013e31820436cb
[18] Koolen PGL, Vargas CR, Ho OA, et al. Does increased experience with tissue oximetry monitoring in microsurgical breast reconstruction lead to decreased flap loss? The learning effect. Plast Reconstr Surg. 2016;137(4):1093–1101. https://doi.org/10.1097/01.prs.0000481071.59025.82
[19] Saad N, Wang H, Karamanos E. Tissue oximetry readings accurately predict late complications in patients undergoing free flap breast reconstruction: exploring the optimal cut point value. J Reconstr Microsurg. 2020;36(7):534–540. https://doi.org/10.1055/s-0040-1710507
[20] Repez A, Oroszy D, Arnez ZM. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg. 2008;61(1):71–77. https://doi.org/10.1016/j.bjps.2007.04.003
[21] Marotz J, Schulz T, Seider S, et al. 3D-perfusion analysis of burn wounds using hyperspectral ihyperspectral imaging. Burns. 2021;47(1):157–170. https://doi.org/10.1016/j.burns.2020.06.001
[22] Newton E, Butskiy O, Shadgan B, et al. Outcomes of free flap reconstructions with near-infrared spectroscopy (NIRS) monitoring: a systematic review. Microsurgery. 2020;40(2):268–275. https://doi.org/10.1002/micr.30526
[23] Kwasnicki RM, Noakes AJ, Banhidy N, et al. Quantifying the limitations of clinical and technology-based flap monitoring strategies using a systematic thematic analysis. Plast Reconstr Surg Glob Open. 2021;9(7):e3663. https://doi.org/10.1097/GOX.0000000000003663
[24] Thiem DGE, Frick RW, Goetze E, et al. Hyperspectral analysis for perioperative perfusion monitoring-a clinical feasibility study on free and pedicled flaps. Clin Oral Investig. 2021;25(3):933–945. https://doi.org/10.1007/s00784-020-03382-6
[25] Schulz T, Marotz J, Stukenberg A, et al. Hyperspectral imaging for postoperative flap monitoring of pedicled flaps. Handchir Mikrochir Plast Chir. 2020;52(4):316–324. https://doi.org/10.1055/a-1167-3089
[26] Thiem DGE, Romer P, Gielisch M, et al. Hyperspectral imaging and artificial intelligence to detect oral malignancy – part 1 – automated tissue classification of oral muscle, fat and mucosa using a light-weight 6-layer deep neural network. Head Face Med. 2021;17(1):38. https://doi.org/10.1186/s13005-021-00292-0
[27] Urbanos G, Martin A, Vazquez G, et al. Supervised machine learning methods and hyperspectral imaging techniques jointly applied for brain cancer classification. Sensors (Basel). 2021;21(11):3827. https://doi.org/10.3390/s21113827
[28] Dremin V, Marcinkevics Z, Zherebtsov E, et al. Skin complications of diabetes mellitus revealed by polarized hyperspectral imaging and machine learning. IEEE Trans Med Imaging. 2021;40(4):1207–1216. https://doi.org/10.1109/TMI.2021.3049591