ORIGINAL RESEARCH ARTICLE
Short term treatment of secondary lymphedema with hyaluronidase injections reduces mouse hindlimb lymphedema
Farima Dalaeia,b, Amar Bucana,b, Alexander Wiinholta,b, Mads Gustaf Jørgensena,b, Christian Rønn Hansenc,d, Christina Baund,e, Svend Hvidstend,e, Eva Kildall Hejbøld,f, Henrik Daa Schrøderd,f and Jens Ahm Sørensena,b
aResearch Unit of Plastic Surgery, Department of Plastic Surgery, Odense University Hospital, Odense, Denmark; bClinical Institute, University of Southern Denmark, Odense, Denmark; cLaboratory of Radiation Physics, Odense University Hospital, Odense, Denmark; dInstitute of Clinical Research, University of Southern Denmark, Odense, Denmark; eDepartment of Nuclear Medicine, Odense University Hospital, Odense, Denmark; fDepartment of Pathology, Odense University Hospital, Odense, Denmark
Lymphedema is a common complication following breast cancer treatment with axillary lymphadenectomy and radiotherapy. Currently, there is no curative treatment for this disease, hence there is a need for new therapeutic suggestions. The aim of this study was to investigate the effect of hyaluronidase (HYAL) injections after inducing hindlimb lymphedema in 36 female C57BL/6 mice. HYAL injections were administered every second day for 14 days in three groups: (1) HYAL for 1 week followed by saline for 1 week, (2) HYAL for 2 weeks, and (3) saline injections for 2 weeks. Volume of the lymphedema limb was weekly assessed with micro-computed tomography (µ-CT) scans for a total course of 6 weeks. Lymph vessel morphometry was assessed in the end of the study after staining cross-sections of the hindlimb for anti-LYVE-1 blindly. Lymphatic function was assessed by lymphoscintigraphy to assess lymphatic clearance. There was a significant reduction of the volume of lymphedema in mice treated with HYAL-7 compared with mice treated with HYAL-14 (p < 0.05) and saline (p < 0.05). No differences were detected in lymph vessel morphometry and the lymphoscintigraphy between groups. Short-term treatment with HYAL-7 might be a potential therapeutic suggestion for secondary lymphedema induced in mouse hindlimbs. In the future, clinical studies are needed to investigate the potential of HYAL treatment in human beings.
KEYWORDS: Secondary lymphedema; hyaluronidase; hyalase; HYAL; treatment of secondary lymphedema; treatment of lymphedema; animal model
Citation: Journal of Plastic Surgery and Hand Surgery 2023; 58: 40–47. DOI: https://doi.org/10.2340/jphs.v58.7791.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 15 November 2022; Accepted: 2 May 2023; Published: 20 June 2023
CONTACT Farima Dalaei farima.dalaei@gmail.com Department of Plastic Surgery, Odense University Hospital and University of Southern Denmark, J.B. Winsløws Vej 4, Entrance 20, Penthouse 2. floor, 5000 Odense C, Denmark.
Competing interests and funding: The authors report there are no competing interests to declare.
This study was funded by following independent grants: Dagmar Marshalls Foundation, Aase and Ejnar Danielsens Foundation, Andersen-Isted Foundation, Jascha-Foundation, Else and Mogens Wedell-Weddelsborgs Foundation and Kai Lange and Gunhild Kai Lange Foundation.
Introduction
Approximately 10–30% of women diagnosed with breast-cancer will develop lymphedema due to the mastectomy, axillary lymphadenectomy and/or irradiation therapy [1,2]. Currently, there is no curative treatment of lymphedema. It is primarily treated with lifelong conservative compression garments and physiotherapy [3]. Hence, there is an obvious need for a better molecular understanding of this disease and new therapeutic suggestions.
Secondary lymphedema is a complex condition characterized by disruption of previously normal lymphatics, causing stagnation of extracellular fluid [4,5]. As the condition progresses, the initial lymphatic stasis leads to inflammation, fibrosis, and fat deposition in the interstitium. This results in a radical enlargement and permanent hardening of the affected limb, further inhibiting the lymphatic circulation [6,7]. Once the chronic state has established, it is hardly reversed [8]. Some studies suggest that there is an accumulation of hyaluronic acid (HA) in human lymphedema [9–11]. HA is known as a major component of the extracellular matrix (ECM), where it increases the viscosity and acts as a barrier to the diffusion of free solutes [12,13]. The role of HA in lymphedema limbs is unknown and data are limited [9,10,14].
The enzyme hyaluronidase (HYAL) hydrolyzes HA, thereby decreasing the viscidity of the ECM and reducing the resistance to fluid absorption [15]. Fluids are more easily diffused and more quickly absorbed into the general circulation [13]. This action of HYAL has been widely used to accelerate the diffusion and absorption of subcutaneously injected fluids and drugs [16,17]. However, only limited studies have investigated the potential of HYAL as a therapeutic agent preventing lymphedema [15]. This study aimed to investigate the impact of HYAL injections on alleviating hindlimb lymphedema in mice over two distinct treatment periods: the first comprising one week of HYAL injections followed by one week of saline injections, and the second involving two consecutive weeks of HYAL injections. The two treatment groups will be compared to a control group that only receives saline injections.
Materials and methods
The Danish National Animal Inspectorate approved the study (2018-15-0201-01445) and it was reported according to the ARRIVE Guidelines 2.0 [18]. For the use of HYAL, we obtained compassionate use permit by the Danish Medicines Agency (Drug-ID 27415609215).
Animals
Thirty-six 9-week-old female inbred C57BL/6 mice weighing between 16 and 18 g (Janvier Labs, Le Genest-Saint-Isle, Saint-Berthevin Cedex, France) were used in this study. All mice were acclimatized for 7 days in groups of eight prior to study commencement. In the whole study period, all mice were maintained on a normal 12-h day-night cycle at 21°C with a humidity of 45 to 55% and fed a standard diet and water ad libitum. Animals were anesthetized before and during each procedure with a subcutaneous injection of fentanyl 788 µg/kg, fluanisone 25 mg/kg and midazolam 12.5 mg/kg. To prevent hypovolemia during surgery 0.5 mL saline was administered subcutaneously before surgery. Ophthalmic ointment (Viscotears) was also administered. Postoperatively, the mice were housed individually and received oral analgesic treatment (Buprenorphine, 0.2 mg/g) daily for 3 days. All mice were euthanized by cervical dislocation at the end of the study.
Induction of lymphedema
Lymphedema was induced in the right hindlimb of mice according to a previously described model, consisting of three separate procedures: radiation prior to surgery, surgery and post-surgical radiation [19]. See Figure 1 for the study design and time points of each procedure. Radiation was emitted from an x-ray instrument (Gulmay D3100, Xstrahl Camberley, United Kingdom) at a dose rate of 5.11 Gy/min (100 kVp, 10 mA, HVL 2.53 Al). Treatment was administered in two fractions with a dose of 10 Gy 7 days before and 4 days after surgical procedure [19]. A 3.0 mm thick rubber-lead sheet enclosed the irradiated area (Ø25 mm).
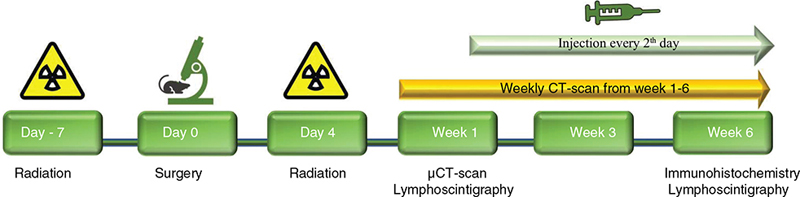
Figure 1. Experimental setup and study design. Study design. Time points of experimental procedures and outcome measurements.
The surgical procedure has previously been described elsewhere [19,20]. Three investigators performed all surgical procedures (F.D., A.W. and A.B.). Briefly, a circumferential incision was made proximal on the midthigh, and the skin was dissected to the knee. A 0.1 mL patent blue V (Guerbet, Cedex, France) was injected between the right second and third toe, visualizing a blue-stained popliteal lymph node, two prenodal and one postnodal lymphatic vessels along the ischiatic vein see Figure 2. The stained lymphatic vessels were tied with 10-0 nylon suture. The popliteal and inguinal lymph nodes were resected along with their fat pads. The procedure was concluded with a circumferential suturing of the skin edges down to the muscle fascia with 6-0 nylon suture. To constrain the superficial lymphatic-flow and mimic the human wound healing process, a wound gap of 2 mm was left out when suturing the skin to muscle fascia, see Figure 2 [19]. Finally, antibiotic ointment was applied at the wound gap to reduce the risk of infection.
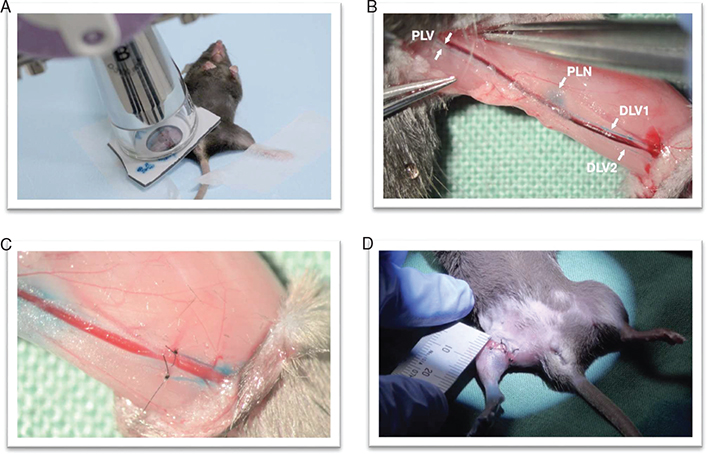
Figure 2. Lymphedema model (19). (A) Radiation prior to surgery. (B) Injection with patent V blue, visualizing the lymph vessels that will be ligated (PLV, DLV1 and DLV2) and the popliteal lymph node (PLN) that will be removed. The inguinal lymph node is not depicted in this figure. (C) Ligated DLV1 and DVL2 and positive milking test, shows inhibited lymph flow through the DLV1 and 2. (D) Circumferential suturing of the skin edges to the muscle fascia leaving a gap of 2 mm. PLV = Proximal lymph vessel, PLN = proximal lymph node, DLV = distal lymph vessel.
Hyaluronidase injections
This study consisted of three treatment groups, with 12 mice in each group served in this study. HYAL-7 mice were treated with HYAL for 7 days followed by saline for 7 days, HYAL-14 mice were treated with HYAL for 14 days, and SALINE mice (control group) were treated with saline for 14 days, see Figure 3 for the injection technique of the mice.
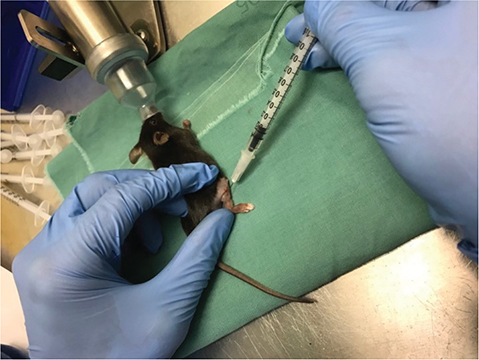
Figure 3. Injection technique of the treatments. All injections were administered every second day in 14 days. The HYAL-7 mice (n = 11) were treated with hyaluronidase for 7 days followed by saline injection for the remaining 7 days. HYAL-14 mice (n = 11) were treated with hyaluronidase for the whole period. The control group, SALINE mice (n = 12) were treated with natural saline.
Hyaluronidase 1.500 IU (Hyaluronidase, Wockhardt United Kingdom) was dissolved in 0.5 mL Saline (Sodium Chloride solution 9 mg/mL, B Braun Medical, Denmark) in 0.5 mL syringes with 27-gauge needle and subcutaneous injected in the right hindlimb of the mice every second day. The control group was injected with 0.5 mL saline subcutaneously in the right hindlimb. Before every injection, mice were anesthetized with a mixture of 1.5–2.0% isoflurane (ScanVet, Denmark) and 100% oxygen and placed in a prone position.
Sample size, randomization, and blinding
Thirty-six mice were included in total based on a power calculation of 80% power and a significance level of 5% derived from a previously described lymphedema model [19]. With a volume of 196 mm3 and a standard deviation of 9.7 mm3, a sample size of 10 mice in each treatment arm (30 in total), a volume reduction of at least 13.8 mm3 would be detectable. Two additional mice were added to each group in case of preterm euthanization of mice due to ethical reasons. Mice were randomly allocated into three groups. In the whole study period and when assessing the results, two investigators (F.D. and A.B.) were blinded. One investigator (A.W.) injected HYAL and saline during the study period and was not part of any data analysis. A.B. and F.D. performed all analyses and were both blinded.
Micro-computed tomography and Lymphoscintigraphy
All mice underwent weekly micro-computed tomography (µ-CT) scans of both hindlimbs for repetitive volume assessments. Small-animal single-photon emission computed tomography (SPECT)/CT was performed as in an earlier described model [19]. In brief, mice were scanned on a Siemens INVEON multimodality preclinical scanner (Siemens Preclinical Solutions, Knoxville, TN, USA). The projection was set to 1200 ms and the transaxial field of view was 44 mm. Before every imaging session, mice were anesthetized with a mixture of 1.5–2.0% isoflurane and 100% oxygen and placed in prone position on a heated SPECT/CT animal bed.
Two mice per group (six in total) underwent Technetium 99m human serum albumin (99Tc-HSA) lymphoscintigraphy at weeks 1 and 6 to assess the lymphatic clearance. Mice were injected subcutaneously with a bolus injection of 0.02 mL 99mTc-HSA between each hindlimb’s second and third toe. Doses were approximately 14.9 ± 1.9 MBq for left hindlimb and 15.1 ± 1.5 MBq for right hindlimb (Vasculocis, CIS Bio International, Paris, France) administered using a 0.5 mL 30-gauge syringe (Covidien, Medtronic Minneapolis, USA). The tracer uptake was expected to have reached steady state after 45 min [21]. The SPECT/CT was performed after 45 min and 4 h after injection of 99mTc-HSA and moved around freely between imaging sessions.
Immunohistochemistry
Hindlimbs were fixed in 4% paraformaldehyde for 48 h. The hindlimbs were cut 10 mm distal to the heel and decalcified in 4.0 M formic acid/0.5 M sodium formate for 24 h before paraffin embedding. To detect lymphatic vessels, 3 μm thick sections were attained and stained with anti-LYVE-1 (ab33682, Abcam, Cambridge, United Kingdom). Tris-EGTA buffer (pH 9.0) at 60°C overnight was used to achieve antigen retrieval. Specimens were incubated with primary antibodies (1:10000) for 60 min at room temperature. Envision + horseradish peroxidase-labeled polymer (K4003, Dako, Agilent, Glostrup, Denmark)/DAB+ was used as a detection system.
Data analysis
SPECT/CT
Data analysis of the SPECT/CT fused images was performed with INVEON Research Workplace software, version 4.2 (IRW, Siemens Healthcare, Ballerup, Denmark). The lymphatic clearance of the operated- and non-operated limb was calculated from lymphoscintigraphy scans made 45 min and 4 h after tracer injection. Volumes of interest were defined around the injection site. The activity decline from the injection site was presumed to follow a monoexponential function e-kt. The removal rate was calculated as 100%k (%/min) [19].
Micro-computed tomography
The hindlimbs underwent volumetric measurements and were calculated from acquired CT images. To standardize the measurements, the distal tibiofibular joint was localized in three-dimensional axial images using a previously described method [19] and functioned as a proximal landmark. The volume of the hindlimb distally from the tibiofibular joint was then calculated using the simple thresholding techniques (Siemens IRW software). All voxels within the Hounsfield-interval of −500 to 4000 units were included in the hind limb volume. The volume of the operated and treated hindlimb was compared with the non-operated limb throughout the entire study period for each mouse in each group.
Lymph vessel morphometry
Sectioned images of both hindlimbs of each mouse were attained using Nanozoomer Digital Pathology (Hamamatsu Photonics, Boston, MA, USA) and analyzed with NDP viewer. For each sectioned image, the region of interest was enclosed manually in two dimensions within the dorsal footpad, laterally by the three middle metatarsals. The lumen of all Lyve-1-stained lymph vessels within the enclosed area were then obtained using the Freehand Region Function. Lastly, the total quantity of the lumen of the lymph vessels for each footpad were calculated using Visiopharm Vision Software. Lymph vessel dilation and the number of lymph vessels were estimated with LYVE-1 morphometry of the dorsal footpad. The total lymph vessel lumen of each footpad was divided with the quantity of lymph vessels.
Statistical methods
All data were analyzed using STATA 15 (Stata Statistical Software: Release 15. College Station, Tex; StataCorp LP: 2015) and Prism 6.0 (GraphPad Software, La Jolla, California).
For each week and each group, the mean volume of the lymphedema limb and the control limb were measured with μ-CT scans and weekly volume changes from baseline were calculated (volume of operated limb – volume of control limb). The change of volume from baseline was calculated for all 6 weeks. Normality was tested with Q-Q plots and Shapiro–Wilk test, indicating that these data were non-normally distributed. Hence, data are presented in medians using non-parametric testing. Kruskal–Wallis test was used to assess the significant difference between change of excess volume in each group. Mann–Whitney U-test was used to assess whether there was a statistical difference between two of the three groups (A-B, A-C, and B-C). All values were reported as a median and interquartile range (IQR) between the first and third quartile (Q1 and Q3).
To compare the difference in lymph vessel immunohistochemistry and lymphoscintigraphy two-way test of variance was used to compare the operated and control hindlimbs within each group with Sidak’s multiple-comparison test for parametric values. All values were reported as mean ± standard deviations (SD). A p-value < 0.05 was considered significant.
Results
All operated limbs presented with postoperative swelling in varying degrees. The operated hindlimb was compared with the non-operated hindlimb throughout the entire study period for each mouse in each group. During the course of the study, two mice died. One mouse from the group receiving a one-week HYAL treatment (HYAL-7) died during the second week, and another mouse from the group receiving a two-week HYAL treatment (HYAL-14) died in the sixth week following the lymphoscintigraphy procedure.
Volume assessment
The results of lymph volume are summarized in Table 1 and graphed in Figure 4. There was a significant difference between HYAL-7 and HYAL-14 in week 4r (p < 0.005) and week 6 (p < 0.05), and between HYAL-7 and SALINE in weeks 4–6 (p < 0.05). There were no differences between HYAL-14 and SALINE in all 6 weeks.
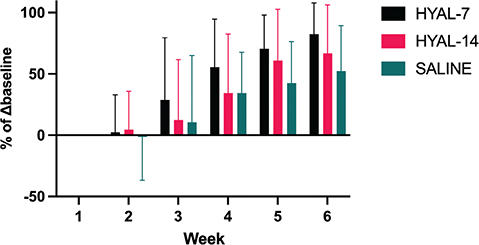
Figure 4. Percentage of baseline change of volume. Δbaseline = change from baseline, HYAL-7 = hyaluronidase for 7 days followed by saline for 7 days, HYAL-14 = hyaluronidase for 14 days, SALINE = saline for 14 days. Percentage of baseline change. A change of baseline of 100% means that the mouse has recovered completely. Baseline is week 1 volume difference between the operated and the non-operated (control) limb as the mice did not receive treatment at this time point. Thereby calculating a baseline excesses volume (lymphedema). The difference between excess volume (lymphedema) each week and baseline was calculated for the rest of the 6 weeks. Then, the percentage was calculated based on the baseline.
Lymph vessel morphometry
The results of the total vessel lumen of each footpad and the mean lumen of lymph vessels are summarized in Table 2. There were no differences in total lymph vessel area between the three groups. The mean lymph vessel lumen was 130.6(±76.9) μm2 in HYAL-7 limbs, 119.1(±108.1) μm2 in HYAL-14 limbs and 144.4(±74.3) μm2 in saline treated limbs, p = 0.07.
Lymphoscintigraphy
Only two mice from each treatment group were included for lymphoscintigraphy to illustrate inhibited lymph flow at baseline and the change at the end of the study. The results are summarized in Figure 5. There was no detectable difference in lymphatic clearance between the three groups when only including six mice in total.
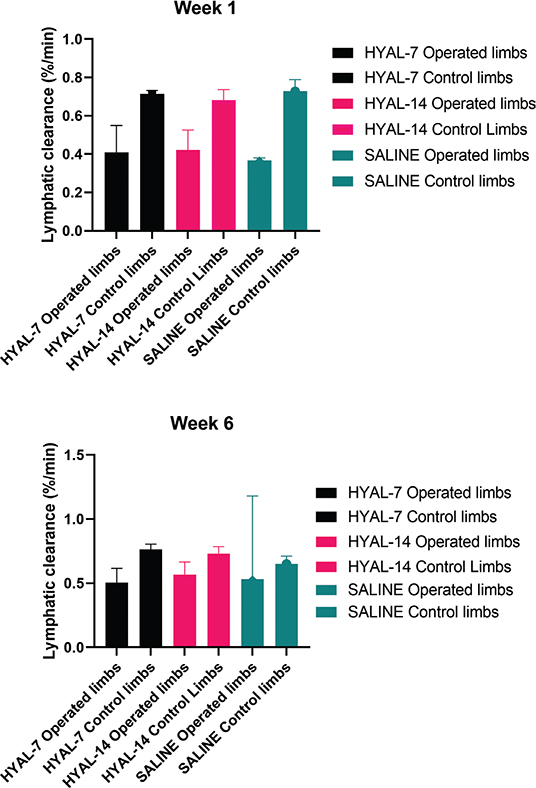
Figure 5. Lymphatic clearance. HYAL-7 = hyaluronidase for 7 days followed by saline for 7 days, HYAL-14 = hyaluronidase for 14 days, SALINE = saline for 14 days. Mean lymphatic clearance % per min. (± standard deviation) for each group after 45 min and 4 h week 1 and week 6.
Discussion
In this study, we investigated the effect of HYAL injection on a lymphedema mouse hindlimb model over the course of 6 weeks. Three treatment groups of 12 mice in each group were injected every second day for 14 days, respectively, with HYAL for 14 days, HYAL for 7 days followed by saline for 7 days, and saline for 14 days. The lymphedema limb was compared with the non-operated hindlimb with weekly μ-CT scans, in the end of the study with lymphoscintigraphy, and lymph vessel morphometry. There was a significant reduction of lymphedema volume of HYAL-7 mice at weeks 4–6 compared with mice treated with saline and between HYAL-7 and HYAL-14 at weeks 4 and 6. There were no differences between mice treated with saline and HYAL-14. Lymph vessel morphometry showed no significant differences between the three treatment groups. The mean lumen of lymph vessels in the dorsal footpad was smaller in the HYAL-14 compared with HYAL-7 and saline (p = 0.07), although not significant. Lymphoscintigraphy showed no differences between the three groups.
Lymphedema volume was significantly reduced in mice treated with HYAL-7 compared with HYAL-14 and saline. Surprisingly, there were no difference between HYAL-14 mice and SALINE. This might indicate a treatment window of 7 days of HYAL treatment as HYAL injections for subsequently 7 more days did not further reduce the volume of lymphedema. Correspondingly to this study, in a mouse tail lymphedema model Roberts et al. found that HA was upregulated on days 5, 15 and 20. On day 5 the increased HA resulted in tail swelling, and from day 10 the diameter of the tail gradually decreased. This is consistent with our findings in the HYAL-14, where the lymphedema volume increases between weeks 1 and 2. However, in contrast to our study, Roberts et al. initiated injections with HYAL from day 12 to 18 postoperatively and found HYAL worsening the edema. This might also advocate for a treatment window of HYAL treatment [22]. To our knowledge, this is the first study to investigate the effect of HYAL in two treatment durations. The optimum treatment window, number of injections and dilution of HYAL should be further investigated in future studies.
We have previously shown that the results of the μ-CT scans are extremely precise. Wiinholt et al. investigated the inter- and intra-rater agreement of the μ-CT scans, showing a low risk of measurement bias [23]. Bucan et al. investigated the interrater agreement of μ-CT scans, the electronic caliper of paw thickness, and plethysmometer in the measurement of lymphedema in mouse hindlimb, also showing that μ-CT scans had the highest interrater agreements and had a low risk of measurement bias [24]. Therefore, the μ-CT scan is a reliable and validated measurement modality.
Similar to our findings, Roh et al. found reduced lymphedema volume with hyaluronidase injection. They also found increased LYVE-1 expression in the immunohistochemistry, suggesting that HYAL promoted lymphangiogenesis in the lymphedematous limb and better lymphatic drainage measured with lymphoscintigraphy [14]. However, in our study, neither the lymphoscintigraphy nor immunohistochemistry showed any difference between the three groups. In the lymphoscintigraphy, only two mice served in each group. Therefore, the results should be interpreted cautiously due to the risk of a type one error. Cross-sections of fixed hindlimbs were stained for LYVE-1 antibodies. The results of the immunohistochemistry were bound to high variability depending on which each axial cut/cross-section of the fixed hindlimb that were chosen for random analysis. Also, there was great uncertainty in the analysis process of the LYVE-1-stained lymph vessels, as non-specific cells were stained as well in the process, making it difficult to differentiate the lymph vessels from other stained cells. The results of the immunohistochemistry should, therefore, also be interpreted carefully. There were no differences detected in the total lymph vessel lumen and the mean lumen of lymph vessels between the three groups. The observation of smaller and more numerous lymph vessels in the groups treated with HYAL compared to the saline group could potentially be attributed to the process of lymphangiogenesis occurring in the treated groups. However, this hypothesis warrants further research to be definitively confirmed.
Of the three measurement modalities μ-CT scans, immunohistochemistry, and lymphoscintigraphy the results of the μ-CT scans were superior to the two others. Clinically, lymphedema is characterized by volume increase and stage depending on the degree of swelling [25]. The primary goal of lymphedema treatment should foremost be to decrease the volume of the edema. Thus, the number, size, and function of the lymph vessels become secondary considerations if the treatment does not effectively reduce the size/volume of the limb.
Microneedling of the skin is known to result in a regenerative response. Both animal models and in vitro examination of human tissue have shown that microneedling creates microchannels and microwounds, inducing wound healing cascade and neovascularization [262–28]. Such a regenerative response could potentially have reduced the lymphedema swelling in all groups, including the control saline group. Particularly as the injections were given every second day for 14 days in all groups.
No studies have investigated the optimal dilution of HYAL or the ideal number of injections. Roh et al. showed reduced lymphedema volume and lymphangiogenesis; however, they did not report the concentration of HYAL [14]. In a mouse tail lymphedema model Jeong et al. used an HYAL concentration of 150 IU/0.1 mL, only injecting once 9 days postoperatively. In accordance with our study Jeong et al. also showed reduced lymphedema volume of the tail; however, they also found increased LYVE-1 after HYAL injection and better lymphatic function with lymphoscintigraphy [29]. Nekoroski et al. used an HYAL gel application of 30 µL recombinant human HYAL 24 h preoperatively, 2 and 12 days postoperatively, which also showed reduced tissue fluid content and edema resolution of the murine tail [30]. As in our study, Cho et al. used a concentration of 1500 IU of HYAL, however, dissolved in 100 µL saline and injected into the hindlimb of mice with three injections of HYAL. Seven days after the third HYAL injection, they found the swelling of the lymphedematous tissue to have reduced to normal, as in the control group. Furthermore, histologically they found significantly reduced dermal thickness and fibrotic area compared with the control group. Their results are equivalent to our HYAL-7, indicating that maybe a treatment of three injections with 1500 IU HYAL might be the optimum. However, this needs further investigation in future studies [10].
One of the strengths of this study is the blinded, randomized design with a large sample size compared with other rodent studies and a duration much longer than most animal studies [10,14,22,29,30]. Another strength of this study is the lymphedema measurement with µ-CT scans, showing extremely high precision and low risk of bias compared with conventional measuring modalities [23].
However, a potential limitation of any mice lymphedema model is maintaining lymphedema for a longer period, especially in the whole study period for investigation of different treatments of lymphedema [14]. Furthermore, the immunohistochemistry could be bound to bias, as it was difficult to differentiate the different died cell types. The surgical procedures were carried out by three different surgeons. Although they all have comparable surgical experience, the potential for bias exists due to the variability in individual surgical techniques. In order to adjust for this, mice were randomly allocated into three different groups, minimizing this potential risk of bias. The primary limitation of this study and all rodent lymphedema models is whether these models can be used to mimic the pathology of lymphedema in human beings. The lymphatic system, immunology and regenerability of humans and mice are very different, and it is uncertain whether HYAL will have the same impact on human lymphedema or not [31]. Hence, future studies are needed to investigate the effect of HYAL in clinical trials.
Conclusion
The injection with HYAL-7 significantly reduces the volume of mice lymphedema in the hindlimb of mice compared with a control group injected with saline and HYAL-14. There were no differences between mice treated with HYAL-14 and saline. This study suggests that the enzyme HYAL can be a promising candidate for the treatment of secondary lymphedema when treated for 7 days. However, further studies are needed to investigate the differences in HYAL treatment durations and whether HYAL is a potential treatment of secondary lymphedema in humans.
Acknowledgments
The authors are very thankful of Peter Bollen PhD, head of section, and the animal technicians at the Biomedical Laboratory for their help with the animals in this study.
ORCID
Farima Dalaei:  https://orcid.org/0000-0003-2305-7758
https://orcid.org/0000-0003-2305-7758
Amar Bucan:  https://orcid.org/0000-0003-2775-8463
https://orcid.org/0000-0003-2775-8463
Alexander Wiinholt:  https://orcid.org/0000-0003-4760-3549
https://orcid.org/0000-0003-4760-3549
Mads Gustaf Jørgensen:  https://orcid.org/0000-0002-8755-3446
https://orcid.org/0000-0002-8755-3446
Christian Rønn Hansen:  https://orcid.org/0000-0001-5716-6069
https://orcid.org/0000-0001-5716-6069
Christina Baun:  https://orcid.org/0000-0002-5199-9200
https://orcid.org/0000-0002-5199-9200
Svend Hvidsten:  https://orcid.org/0000-0002-8857-4521
https://orcid.org/0000-0002-8857-4521
Eva Kildall Hejbøl:  https://orcid.org/0000-0002-3462-321X
https://orcid.org/0000-0002-3462-321X
Henrik Daa Schrøder:  https://orcid.org/0000-0001-7588-235X
https://orcid.org/0000-0001-7588-235X
Jens Ahm Sørensen:  https://orcid.org/0000-0003-4903-0094
https://orcid.org/0000-0003-4903-0094
References
[1] DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. https://doi.org/10.1016/S1470-2045(13)70076-7
[2] Armer JM, Ballman KV, McCall L, Ostby PL, Zagar E, Kuerer HM, et al. Factors associated with lymphedema in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection. JAMA Surg. 2019;154(9):800–809. https://doi.org/10.1001/jamasurg.2019.1742
[3] Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;159(1):1–14. https://doi.org/10.1007/s10549-016-3919-4
[4] Weiler MJ, Cribb MT, Nepiyushchikh Z, Nelson TS, Dixon JB. A novel mouse tail lymphedema model for observing lymphatic pump failure during lymphedema development. Sci Rep. 2019;9(1):10405. https://doi.org/10.1038/s41598-019-46797-2
[5] Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci (Lond). 1993;85(6):737–746. https://doi.org/10.1042/cs0850737
[6] Ryan TJ. Lymphatics and adipose tissue. Clin Dermatol. 1995;13(5):493–498.
[7] Hespe GE, Nores GG, Huang JJ, Mehrara BJ. Pathophysiology of lymphedema-Is there a chance for medication treatment? J Surg Oncol. 2017;115(1):96–98. https://doi.org/10.1002/jso.24414
[8] Beederman M, Chang DW. Advances in surgical treatment of lymphedema. Arch Plast Surg. 2021;48(6):670–677.
[9] Liu NF, Zhang LR. Changes of tissue fluid hyaluronan (hyaluronic acid) in peripheral lymphedema. Lymphology. 1998;31(4):173–179.
[10] Cho S, Roh K, Park J, Park YS, Lee M, Cho S, et al. Hydrolysis of hyaluronic acid in lymphedematous tissue alleviates fibrogenesis via TH1 Cell-mediated cytokine expression. Sci Rep. 2017;7(1):35. https://doi.org/10.1038/s41598-017-00085-z
[11] Steen EH, Short WD, Li H, Parikh UM, Blum A, Templeman N, et al. Skin-specific knockdown of hyaluronan in mice by an optimized topical 4-methylumbelliferone formulation. Drug Deliv. 2021;28(1):422–432. https://doi.org/10.1080/10717544.2021.1886376
[12] Ogston AG, Sherman TF. Effects of hyaluronic acid upon diffusion of solutes and flow of solvent. J Physiol. 1961;156:67–74.
[13] Brix B, Apich G, Rössler A, Walbrodt S, Goswami N. Effects of physical therapy on hyaluronan clearance and volume regulating hormones in lower limb lymphedema patients: a pilot study. Sci Prog. 2021;104(1):36850421998485. https://doi.org/10.1177/0036850421998485
[14] Roh K, Cho S, Park JH, Yoo BC, Kim WK, Kim SK, et al. Therapeutic effects of hyaluronidase on acquired lymphedema using a newly developed mouse limb model. Exp Biol Med (Maywood). 2017;242(6):584–592.
[15] Magistro CM. Hyaluronidase by iotophoresis in the treatment of edema. Phys Ther. 1964;44:169–175. https://doi.org/10.1093/ptj/44.3.169
[16] Huang X, Han S, Chen Z, Zhao L, Wang C, Guo Q, et al. Layered double hydroxide modified with deoxycholic and hyaluronic acids for efficient oral insulin absorption. Int J Nanomed. 2021;16:7861–7873. https://doi.org/10.2147/IJN.S323381
[17] Cañibano-Hernández A, Saenz Del Burgo L, Espona-Noguera A, Orive G, Hernández RM, Ciriza J, et al. Hyaluronic acid enhances cell survival of encapsulated insulin-producing cells in alginate-based microcapsules. Int J Pharm. 2019;557:192–198.
[18] Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. https://doi.org/10.1371/journal.pbio.3000410
[19] Jorgensen MG, Toyserkani NM, Hansen CR, Hvidsten S, Baun C, Hejbol EK, et al. Quantification of chronic lymphedema in a revised mouse model. Ann Plast Surg. 2018;81(5):594–603.
[20] Wiinholt A, Jørgensen MG, Bučan A, Dalaei F, Sørensen JA. A Revised method for inducing secondary lymphedema in the hindlimb of mice. J Vis Exp. 2019;153:e60578. https://doi.org/10.3791/60578-v
[21] Modi S, Stanton AW, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5(3):183–202. https://doi.org/10.1089/lrb.2007.5306
[22] Roberts MA, Mendez U, Gilbert RJ, Keim AP, Goldman J. Increased hyaluronan expression at distinct time points in acute lymphedema. Lymphat Res Biol. 2012;10(3):122–128. https://doi.org/10.1089/lrb.2012.0001
[23] Wiinholt A, Gerke O, Dalaei F, Bučan A, Madsen CB, Sørensen JA. Quantification of tissue volume in the hindlimb of mice using microcomputed tomography images and analysing software. Sci Rep. 2020;10(1):8297. https://doi.org/10.1038/s41598-020-65214-7
[24] Bucan A, Wiinholt A, Dalaei F, Gerke O, Hansen CR, Sørensen JA. Microcomputed tomography versus plethysmometer and electronic caliper in the measurements of lymphedema in the hindlimb of mice. Sci Rep. 2022;12(1):12267. https://doi.org/10.1038/s41598-022-16311-2
[25] Lafuente H, Jaunarena I, Ansuategui E, Lekuona A, Izeta A. Cell therapy as a treatment of secondary lymphedema: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12(1):578. https://doi.org/10.1186/s13287-021-02632-y
[26] Juhasz MLW, Cohen JL. Microneedling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997–1003.
[27] El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8(7):36–42.
[28] Busch KH, Aliu A, Bender R, Walezko N, Aust MC. [Medical needling: effect on skin tension and elasticity of hypertrophic burn scars]. Handchir Mikrochir Plast Chir. 2019;51(5):384–393. https://doi.org/10.1055/a-0996-8572
[29] Jeong HJ, Roh KH, Kim GC, Kim YO, Lee JH, Lee MJ, et al. Hyaluronidase treatment of acute lymphedema in a mouse tail model. Lymphology. 2013;46(4):160–172.
[30] Nekoroski T, Paladini RD, Sauder DN, Frost GI, Keller GA. A recombinant human hyaluronidase sustained release gel for the treatment of post-surgical edema. Int J Dermatol. 2014;53(6):777–785. https://doi.org/10.1111/ijd.12304
[31] Hadrian R, Palmes D. Animal models of secondary lymphedema: new approaches in the search for therapeutic options. Lymphat Res Biol. 2017;15(1):2–16. https://doi.org/10.1089/lrb.2016.0015