ORIGINAL RESEARCH ARTICLE
Recurrence of non-muscle invasive bladder carcinoma after transurethral resection with hexaminolevulinate photodynamic diagnosis or regular cystoscopy
Fokke Jan Sebastiaan Hoogeveena , Marcus Hendrikus Blankerb
, Marcus Hendrikus Blankerb , Evelyne Clara Carolyne Cauberga and Martijn Geert Steffensa
, Evelyne Clara Carolyne Cauberga and Martijn Geert Steffensa
aDepartment of Urology, Isala Hospital, Zwolle, The Netherlands; bDepartment of General Practice and Elderly Care Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
ABSTRACT
Objectives: To compare the recurrence of non-muscle invasive bladder carcinoma (NMIBC) after transurethral resection employing cystoscopy with hexaminolevulinate-based photodynamic diagnosis (PDD) or with standard white light.
Patients and methods: We included patients with newly suspected NMIBC in this retrospective cohort study and compared those undergoing transurethral resection by white light cystoscopy (WLC) (2008–2010) and PDD (2010–2012). All patients were treated following established criteria for good quality resection. The primary outcome was the difference in the recurrence rate after 60 months’ follow-up, but we also stratified recurrence by risk groups, as set by the European Organization for Research and Treatment of Cancer. The mean recurrence-free survival was compared between the cohorts. Odds ratios or hazard ratios are reported with their 95% confidence intervals.
Results: The WLC and PDD cohorts comprised 124 and 91 subjects, respectively. There were no significant differences in recurrence rates between the cohorts at 6 months (recurrence rate 9/123; 7.3%), 12 months (17/118; 14.4%) or 60 months (39/102; 38.2%), with odds ratios of 1.23 (CI 0.48–3.25), 1.32 (CI 0.67–2.62) and 1.12 (CI 0.70–1.79), in favour of WLC, respectively. Further analysis showed no significant effect of PDD on either recurrence by risk group or on mean recurrence-free survival (hazard ratio, 1.12 [CI 0.70–1.79]).
Conclusion: We found no relevant differences in the recurrence of NMIBC after the introduction of PDD with hexaminolevulinate compared to standard WLC when used for transurethral resection in our single institution.
KEYWORDS: Cystoscopy; bladder cancer; fluorescence imaging; recurrence; 5-aminolevulinic acid
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 120–125. https://doi.org/10.2340/sju.v58.10160.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 22 February 2023; Accepted: 13 October 2023; Published: 6 December 2023
CONTACT F.J.S. Hoogeveen sebastiaanhoogeveen@gmail.com Department of Urology, Isala Hospital, Dokter van Heesweg 2, 8025 AB, Zwolle, The Netherlands
Competing interests and funding: The authors report no conflicts of interest.
Introduction
Non-muscle invasive bladder cancer (NMIBC) accounts for most bladder cancers and can usually be treated by transurethral resection (TUR) [1]. However, the efficacy of TUR is highly variable, with recurrence rates of up to 78% at 5 years [2]. Early recurrence results from incomplete resection of the primary tumour or the presence of undetected tumours [3]. The current standard approach for TUR involves white light cystoscopy (WLC), which is effective for detecting papillary tumours. However, carcinoma in situ (CIS), small satellite growths or dysplasia can be missed [4].
To improve tumour detection, photodynamic diagnosis (PDD) was developed that uses photoactive porphyrins that accumulate in neoplastic tissue and become fluorescent under blue light [4]. Hexaminolevulinate (HexvixTM; Ipsen, France) is one such photoactive agent that has been shown to increase tumour detection rates by 9.7%–40.2% [5, 6]. The effect of PDD on recurrence rates has therefore gained interest, despite systematic reviews and meta-analyses of randomised controlled trials showing varied results. In 2012, Shen reported no effect on short-term recurrence in a systematic review of different fluorescent agents, including hexaminolevulinate [7]. Shortly thereafter, Yuan reported a significant effect of PDD on the time to first recurrence and on the recurrence-free survival (RFS) at 1 and 2 years [8], whereas Burger reported an insignificant effect on time to recurrence and a significant effect on the recurrence rates for hexaminolevulinate alone [6]. A meta-analysis by Chou in 2017 showed that the use of hexaminolevulinate had a significant effect on recurrence [9]. In 2022, Maisch published a Cochrane review, which suggests favourable impact on recurrence in medium- to high-risk subgroups [10]. Most reviews published have reported the quality of evidence to be low, mainly because of heterogeneity (e.g. trials included both new and recurrent carcinoma), lack of treatment standardisation (e.g. intravesical chemotherapy) and lack of subgroup analysis [7–11]. Nevertheless, despite the inconclusive reports, PDD has been introduced in clinical care. In a prospective single-centre study, Gallagher showed that using PDD routinely in a real-world setting significantly decreased the long-term recurrence of NMIBC. In their research, treatment was standardised with recommended ‘good quality resection’ and the instillation of post-operative mitomycin C (MMC) [10, 11].
In our hospital, PDD replaced WLC in 2010 as the standard method for TUR when treating patients with suspected NMIBC. We therefore sought to confirm the results of Gallagher [12] in our population. In this study, we compared how hexaminolevulinate-based PDD and WLC TUR affected the recurrence of newly presenting NMIBC over a 5-year follow-up period.
Patients and methods
We compared the outcomes of consecutive patients newly presenting with NMIBC and treated in our hospital between January 2008 and December 2012. This resulted in time series for a WLC cohort (from 2008 to PDD introduction) and a PDD cohort (from PDD introduction onwards) that were mutually exclusive. This study duration allowed for a follow-up period of 5 years in all cases. The primary outcome was the time to recurrence. Follow-up was performed by WLC in both groups, according to European Association of Urology (EAU) guidelines [1]. Ethical approval was waived by the local Ethics Committee of the Isala Hospital in Zwolle, the Netherlands.
Before the introduction of PDD, all patients suspected of bladder carcinoma underwent TUR with WLC. Afterwards, only patients with newly suspected NMIBC underwent a standardised TUR procedure with PDD. Eligible patients were identified from our patient directory based on the operation performed, using the CTcueTM software (CTcue, the Netherlands). Patients were excluded if the resection did not meet the good quality resection criteria, PDD was unsuccessful (absence of fluorescent signal), muscle invasive carcinoma was pathologically proven after TUR, malignancy other than urothelial cell carcinoma (UCC) was found or if patients did not undergo adjuvant intravesical chemotherapy or immunotherapy if indicated. Patients with a documented suspicion of muscle invasive carcinoma at initial cystoscopy were not considered for inclusion in any group.
To standardise resection quality, we applied the principles of good quality resection, as suggested by Mariappan, with the exception of cystoscopic bladder mapping, which was not reported in the retrospective data [13]. We applied the following principles [13]: resections carried out by a urologist or by a urology resident under close supervision; all visible and otherwise documented tumours resected; Detrusor muscle resected either en masse or by fractionated resections; and post-operative intravesical MMC instilled. Cystoscopy and bipolar resection were performed in both the PDD and WLC cohorts using a 27 French gage Olympus resectoscope (type A22041). According to the manufacturer’s guidelines, we instilled 50 mL of phosphate-buffered saline containing 85 mg of hexaminolevulinate through the catheter, retained it for 60 min and drained it prior to cystoscopy [14]. A second TURBT was performed if indicated according to EAU guidelines in case of high-risk NMIBC [1].
We retrieved the following data from the medical files of included patients: age at first treatment, histological analysis (e.g. tumour stage, tumour grade and presence of CIS), single post-operative MMC instillation, adjuvant intravesical chemotherapy or immunotherapy, date of first recurrence and date and cause of death. Patient survival was based on data from the Municipal Population Register. Malignancies were divided by European Organization for Research and Treatment of Cancer (EORTC) risk group, with the exception of tumour size, which was not systematically recorded in our data [2].
Analyses were performed using IBM SPSS Version 25 (IBM Corp, USA). We considered a P-value of ≤ 0.05 statistically significant. Descriptive analyses were performed for the baseline characteristics based on their distributions, and differences between the two cohorts were tested using the chi-square test and t-test. The cumulative overall death rate as well as the number of deaths due to confirmed UCC progression were compared between study groups using the chi-square test.
The primary outcome, time until first recurrence, was compared between the two groups over the 5-year follow-up period using Cox proportional logistic regression. We compared the recurrence rates at 6, 12 and 60 months by log-rank test and presented the recurrence rates in survival curves. Study group was entered as the explanatory variable, and censoring was defined as loss to follow-up or death. We checked for imbalanced censoring between the groups by chi-square test of the percentage of censored patients. The resulting data are presented in survival curves with accompanying odds ratios. Univariate Cox regression analyses were then performed to identify factors associated with risk of recurrence: tumour stage, tumour grade, CIS presence, EORTC risk group and receipt of adjuvant intravesical therapy. These confounders were chosen for their putative influence on recurrence according to the EAU guideline on NMIBC [1]. Any variable with a univariate P-value of ≤0.25 was entered into the multivariate logistic regression analysis to calculate the propensity score. Propensity score analysis was performed to adjust for these confounders, using univariable Cox regression analyses with treatment group as the dependent variable and characteristics with a significant imbalance between both groups as independent variables. Any variable with a univariate P-value of ≤ 0.25 was entered into the multivariate logistic regression analysis to calculate the propensity score. Finally, the resulting propensity score was entered in a multivariate Cox regression analysis, using RFS as the dependent variable.
Results
We identified 408 patients who underwent TUR during the study period; of these, 215 (53%) met the inclusion criteria (Figure 1). The baseline characteristics are summarized in Table 1, more T1 tumours were present in the WLC group than the PDD group (P = 0.006) and there was a difference in adjuvant intravesical therapy use between the groups (P = 0.01). In total, 137 patients completed the 5-year follow-up. The number of deaths was higher in the WLC group (n = 54; 44%) than in the PDD group (n = 24; 26%, P = 0.01), but the number of deaths by recurrence was comparable. 38 patients (70%) died recurrence free and 16 (30%) died after recurrence in the WLC group, whereas 17 (71%) died recurrence free and 7 (29%) died after recurrence in the PDD group (P = 0.97). Confirmed UCC progression was present in 14% of deaths, and there were more UCC-related deaths in the WLC group (17%) than in the PDD group (8.3%; P = 0.33).
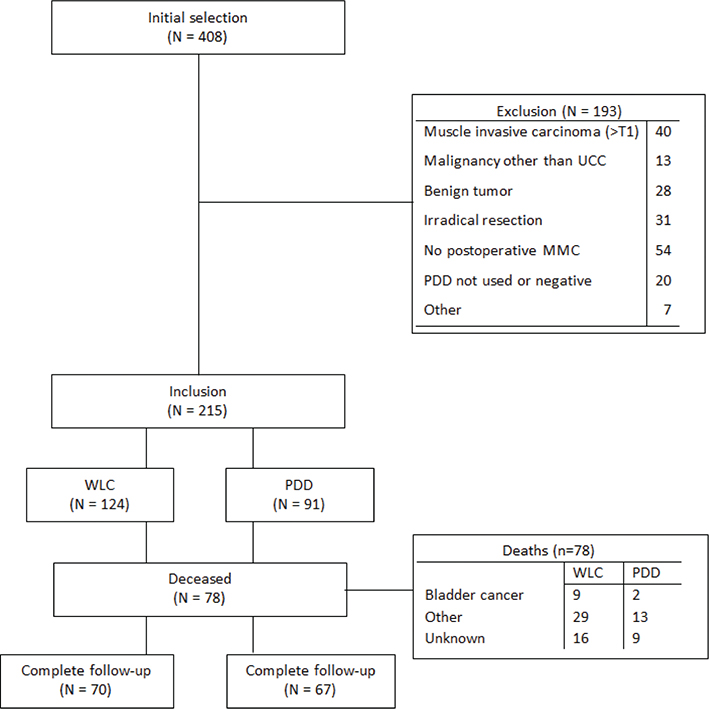
Figure 1. Inclusion flow chart. Benign tumour; inverted papilloma, leiomyoma, inflammation and papillary urothelial neoplasm of low malignant potential. Irradical resection as judged by urologist due to diffuse tumour or intra-operative complications. CIS: carcinoma in situ; EORTC: European Organization for Research and Treatment of Cancer; MMC: mitomycin C; PDD: photodynamic diagnosis; UCC: urothelial cell carcinoma; WLC: white light cystoscopy.
| White light cystoscopy (2008–2010) | Photodynamic diagnosis (2010–2012) | P value* | |
| Demographics | |||
| Number of patients | 124 | 91 | |
| Median age (25–75 percentile) | 73 (64–79) | 71.0 (66–79) | 0.48 |
| Sex (M/F) | 97/27 | 72/19 | 0.8 |
| Tumour characteristics | |||
| T1 | 27 (22) | 6 (6.6) | 0.006 |
| CIS | 11 (8.9) | 8 (8.8) | 0.98 |
| Solitary | 81 (65) | 61 (67) | 0.59 |
| Multifocal (>2) | 43 (35) | 30 (33) | |
| Risk group | |||
| Low (TaG1, solitary, no CIS) | 32 (26) | 27 (30) | 0.52 |
| Intermediate (TaG2, multifocal <7, no CIS) | 46 (37) | 37 (41) | |
| High (T1, G3, CIS, TaG1/G2 + >7 tumours) | 46 (37) | 27 (30) | |
| Adjuvant intravesical therapy | |||
| None or incomplete schedule | 60 (48) | 54 (59) | 0.01 |
| BCG instillations | 20 (16) | 15 (17) | |
| MMC instillations | 44 (36) | 22 (24) | |
| *Pearson Chi-square test. NMIBC: non-muscle invasive bladder carcinoma; TUR: transurethral resection; BCG: Mycobacterium bovis Bacillus Calmette–Guérin; CIS: carcinoma in situ; EORTC: European Organization for Research and Treatment of Cancer; MMC: mitomycin C. |
|||
Recurrence rates at 6 months (7.3%; 9/123), 12 months (14%; 17/118) and 60 months (38%; 39/102) were generally lower in the WLC group than in the PDD group (Table 2), with corresponding odds ratios of 1.23 (95% CI, 0.48–3.25; P = 0.64), 1.32 (95% CI, 0.67–2.62; P = 0.42) and 1.12 (95% CI, 0.70–1.79; P = 0.65), respectively. Recurrence rates at 6, 12 and 60 months did not differ significantly by the EORTC risk group. There was no significant difference between the groups in mean RFS, with a hazard ratio of 1.12 (95% CI, 0.70–1.79; Figure 2).
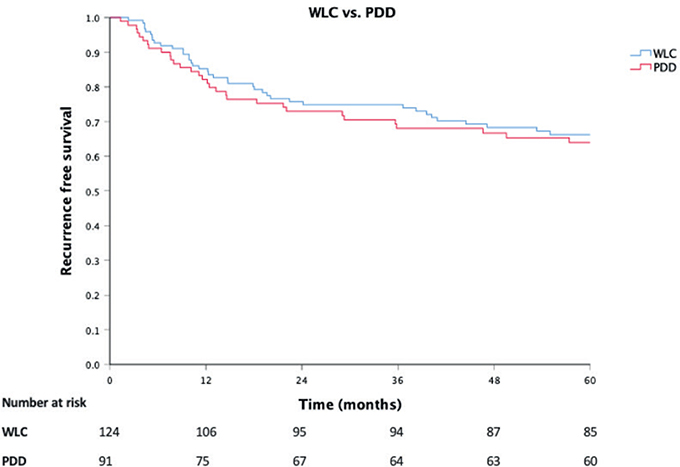
Figure 2. Recurrence-free survival by treatment with white light or photodynamic diagnosis, including number at risk for recurrence. Censoring occurred after loss to follow-up or upon death. Abbreviations: PDD, photodynamic diagnosis; WLC, white light cystoscopy.
Univariate Cox regression revealed that the number of lesions, the tumour stage, the tumour grade, the presence of CIS and the EORTC risk group were all possible confounders (P < 0.25), but adjuvant intravesical chemotherapy was not (Table 3). The Cox regression analyses adjusted for the propensity score, including these characteristics, indicated no significant difference between the WLC and PDD groups (hazard ratio, 1.48; 95% CI, 0.89–2.48).
| Hazard ratio* | 95.0% CI | P value | ||||
| Number of lesions | 1.42 | 0.98–2.07 | 0.064† | |||
| T-Stage | 2.52 | 1.67–3.81 | > 0.001† | |||
| T-Grade | 1.02 | 1.01–1.03 | > 0.001† | |||
| Carcinoma in situ | 2.33 | 1.19–4.56 | 0.013† | |||
| EORTC risk group | 1.41 | 1.03–1.92 | 0.031† | |||
| Adjuvant intravesical therapy | 1.1 | 0.82–1.48 | 0.51 | |||
| *Cox regression analysis. †P < 0.25 was considered as confounder. CI: confidence interval; EORTC: European Organization for Research and Treatment of Cancer. | ||||||
Discussion
This retrospective cohort study did not show any significant difference in recurrence rate or mean recurrence free survival for NMIBC between WLC- and PDD-guided cystoscopy. We believe that this study supplements the existing pool of data from randomised controlled trials and gives a valid representation of how PDD for TUR affects outcomes in a real-world setting, even though the retrospective cohort design of this study may be considered suboptimal.
We would like to address several points. First, at baseline, our WLC group had more T1 tumours (21%) than our PDD group (6.6%). This led to a greater percentage of cases in the high-risk EORTC category in the WLC group (37%) than in the PDD group (30%). Although one might expect such high-risk tumours to recur more often than low- or intermediate-risk tumours, there was no significant difference in recurrence rates between the study groups. It should also be noted that the higher number of T1 tumours in the WLC group may have resulted from selection bias in the PDD group. For example, if patients were suspected of a muscle invasive bladder tumour at initial cystoscopy, they were selected to undergo WLC for TUR and therefore excluded from the study. Given that T1 tumours are more likely to appear macroscopically solid at cystoscopy, they are less likely to have been included in the PDD arm. Another notable baseline characteristic is the substantial proportion (36%) of censored patients during the study’s follow-up period. This prompts the question of whether this population is representative. In our practice, we frequently encounter patients with significant comorbidities who are susceptible to mortality from various causes. In support of our findings, Gallagher also reported a comparable censoring rate of 34%, further suggesting that our study aligns with real-world circumstances [12].
Second, recurrence rates reported in the literature tend to be higher than in this study. For example, in the meta-analysis by Burger, corresponding recurrence rates of 35% and 45% at 12 months were reported (P = 0.006), whereas we showed rates of 18% and 14%, respectively (P = 0.42) [6]. However, it should be noted that most previous reviews have reported on populations, in which both primary and recurrent bladder cancer were included. Given that patients with a history of recurrent disease will be at higher risk of recurrence, one might expect a higher recurrence rate in these studies. Therefore, we chose to include only newly presenting cases of NMIBC and treatment with post-operative single-dose intravesical MMC, consistent with the reports of Heer and Gallagher. RR still varied in those studies, at 36 months. Heer reported recurrence of 42% for PDD and 38% for WLC (P = 0.7), whereas Gallagher reported rates of 39% and 53%, respectively (P = 0.02) [12, 15]. We believe that the strict adherence to the EAU guidelines on adjuvant intravesical chemotherapy or immunotherapy and resections being performed by experienced surgeons or residents under close supervision also contributed to the lower recurrence rate.
Lastly, Chou and Maisch reported on the risks of population and selection heterogeneity in their reviews [9, 10]. By applying strict inclusion and exclusion criteria, we aimed to reduce this heterogeneity (e.g. including only newly presenting patients, having strict rules for resection quality and ensuring adjuvant intravesical therapy). Additionally, we applied propensity regression adjustment in order to adjust for possible confounding covariates. We did not perform propensity sore case matching as this is not superior to using propensity scores in regression analysis as suggested by D’agostino et al. [16].
In conclusion, we found no relevant differences in the recurrence of NMIBC after the introduction of PDD with hexaminolevulinate compared to standard WLC when used for TUR in our single institution. This study adds to the growing body of recent evidence that the use of PDD is less effective in reducing recurrence than previously shown.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
ORCID
F.J.S. Hoogeveen  https://orcid.org/0000-0003-4567-5880
https://orcid.org/0000-0003-4567-5880
M.H. Blanker  https://orcid.org/0000-0002-1086-8730
https://orcid.org/0000-0002-1086-8730
M.G. Steffens  https://orcid.org/0000-0001-7367-643X
https://orcid.org/0000-0001-7367-643X
References
- [1] Babjuk M, Burger M, Compérat EM, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) – 2019 Update. Eur Urol. 2019;76(5): 639–657. https://doi.org/10.1016/j.eururo.2019.08.016
- [2] Sylvester RJ, Van Der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–475. https://doi.org/10.1016/j.eururo.2005.12.031
- [3] Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. BJU Int. 2011; 107(3):396–398. https://doi.org/10.1111/j.1464-410X.2010.09547.x
- [4] Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174(3):862–866. https://doi.org/10.1097/01.ju.0000169257.19841.2a
- [5] di Stasi SM, de Carlo F, Pagliarulo V, et al. Hexaminolevulinate hydrochloride in the detection of nonmuscle invasive cancer of the bladder. Ther Adv Urol. 2015;7(6):339–350. https://doi.org/10.1177/1756287215603274
- [6] Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64(5):846–854. https://doi.org/10.1016/j.eururo.2013.03.059
- [7] Shen P, Yang J, Wei W, et al. Effects of fluorescent light-guided transurethral resection on non-muscle-invasive bladder cancer: a systematic review and meta-analysis. BJU Int. 2012;110(6b):E209–E215. https://doi.org/10.1111/j.1464-410X.2011.10892.x
- [8] Yuan H, Qiu J, Liu L, et al. Therapeutic outcome of fluorescence cystoscopy guided transurethral resection in patients with non-muscle invasive bladder cancer: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(9):1–8. https://doi.org/10.1371/journal.pone.0074142
- [9] Chou R, Selph S, Buckley DI, et al. Review article comparative effectiveness of fluorescent versus white light cystoscopy for initial diagnosis or surveillance of bladder cancer on clinical outcomes: systematic review. J Urol. 2017;197(3):548–558. https://doi.org/10.1016/j.juro.2016.10.061
- [10] Maisch P, Koziarz A, Vajgrt J, Narayan V, Kim MH, Dahm P. Blue vs white light for transurethral resection of non-muscle-invasive bladder cancer: an abridged Cochrane Review. BJU Int. 2022;130(6):730–740. https://doi.org/10.1002/14651858.CD014887.pub2
- [11] Veeratterapillay R, Gravestock P, Nambiar A, et al. Time to turn on the blue lights: a systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur Urol Open Sci. 2021;31:17–27. https://doi.org/10.1016/j.euros.2021.06.011
- [12] Gallagher KM, Gray K, Anderson CH, et al. ‘Real-life experience’: recurrence rate at 3 years with Hexvix®photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC—a prospective controlled study. World J Urol. 2017;35(12):1871–1877. https://doi.org/10.1007/s00345-017-2077-6
- [13] Mariappan P, Rai B, El-Mokadem I, et al. Real-life experience: Early recurrence with Hexvix photodynamic diagnosis-assisted transurethral resection of bladder tumour vs good-quality white light TURBT in new non-muscle-invasive bladder cancer. Urology. 2015;86(2):327–331. https://doi.org/10.1016/j.urology.2015.04.015
- [14] Ipsen Limited. Guideline on Summary of Product Characteristics Hexvix [Internet]. Electronic Medicines Compendium; 2004, p. 1. Available from: https://www.medicines.org.uk/emc/product/4313/smpc [cited 29 April 2023].
- [15] Heer R, Lewis R, Vadiveloo T, et al. A randomized trial of PHOTOdynamic surgery in non–muscle-invasive bladder cancer. NEJM Evid. 2022; 1(10):1–10. https://doi.org/10.1056/EVIDoa2200092
- [16] D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. https://doi.org/10.1002/(SICI)1097-0258(19981015)17:19%3C2265::AID-SIM918%3E3.0.CO;2-B