ORIGINAL RESEARCH ARTICLE
Abandoning testing for asymptomatic microscopic haematuria in Sweden – a long-term follow-up
Per-Uno Malmströma and Truls Gårdmarkb
aDepartment of Surgical Sciences, Uppsala University, Uppsala, Sweden; bDepartment of Clinical Sciences, Danderyd Hospital, Karolinska Institute, Stockholm, Sweden
ABSTRACT
Objectives: To test the hypothesis that the Swedish national policy of abandoning testing for asymptomatic microscopic haematuria (AMH) introduced in 1999 did not adversely affect the prognosis of patients with urinary bladder cancer. Specific aims were to investigate possible effects on (1) Diagnostic delay as represented by stage distribution at diagnosis, (2) Survival and mortality trends, also in comparison to other countries and (3) National health care costs.
Material and methods: The design was an observational study using open sources on patients included in the Swedish National Bladder Cancer Registry 1997–2016. Outcome measures were: Changes in initial tumour presentation during 5 years after the change and long-term relative survival and mortality in comparison to the other Nordic countries. Costs related to investigations were estimated based on the national price lists.
Results: The proportion of patients diagnosed with muscle-invasive bladder cancer decreased following the policy change. The long-term relative 5-year survival increased during the study period. Mortality has remained constant during the period. In comparison to the other Nordic countries, Sweden remains among those with the best outcome despite a more restrictive approach. Cost savings because of the policy change were estimated to be substantial.
Conclusions: Based on open-source registry data, the new restrictive policy was not found to adversely affect the survival of patients with urinary bladder cancer in Sweden. These observations argue against a major negative impact of abandoning further work-up for patients with AMH and may be of use for other countries when revising guidelines. The reduced number of patients undergoing investigation may allow for increased focus and be a relief both for caregivers and the health budget.
KEYWORDS: Epidemiology; public health; hematuria; urinary bladder cancer; health care costs
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 109–114. https://doi.org/10.2340/sju.v58.11142.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 9 March 2023; Accepted: 19 October 2023; Published: 21 Novemeber 2023
CONTACT Per-Uno Malmström per-uno.malmstrom@uu.se Department of Surgical Sciences, Uppsala University, SE751 85 Uppsala, Sweden
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v58.11142
Competing interests and funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Introduction
Recently, several studies have proposed to optimise management of patients with asymptomatic microscopic haematuria (AMH) in an effort to not let ‘gold down the drain’ as stated in one publication [1]. The argument presented is that ‘due to both its relatively high incidence and involved workup, AMH may account for proportionately high health expenditures given the relatively low incidence of underlying malignancy in these patients’. This view was reiterated in a recent systematic review, which found 44 evaluable studies and concluded that the cancer yield with investigating AMH was extremely low [2].
There is consensus that macroscopic haematuria is an alarming sign that requires rapid investigation and thus detecting microscopic haematuria with a test has been advocated as a fast track to early diagnosis in the absence of symptoms. However, the clinical significance of AMH has been controversial, and guidelines on testing have differed from when the test was introduced and up to the present day. No other country or region has to our knowledge gone to the extreme to totally discard testing for AMH as Sweden did in 1999, now more than 20 years ago. This policy was adopted after a review by a panel of experts from the Swedish National Board of Health and Welfare found no evidence in the literature of benefit of investigating patients with asymptomatic microhaematuria [3]. Questions were raised about the risks following the change in policy and case reports on young patients with bladder cancer diagnosed solely on finding AMH were reported [4].
Since then, updated major organisational guidelines have emerged but as pointed out by Linder et al. 2018 ‘the variations in the existing guidelines reflect the absence of level 1 evidence on the subject, as well as differences in relative prioritization across healthcare systems of diagnostic certainty versus fiscal control’ [5]. A development of risk stratification models has been advocated to improve the situation as explained in the American Urological Association 2020 guideline. The new system separates patients into ‘clinically meaningful categories with differing likelihoods of bladder cancer that would justify evaluating risk groups with incremental intensity’ [6]. The majority of patients with AMH will still be recommended to have cystoscopy and upper tract imaging and if negative results have a repeat urine testing after 6 months.
The Swedish approach is thus unique, and it is important to analyse the outcome of the change in policy for the planning of health care in other countries. The aim of this report is to ascertain that the restrictive national policy did not adversely affect the prognosis of patients with newly diagnosed urinary bladder cancer in Sweden. The specific aims were to investigate possible effects on (1) delayed diagnosis as represented by initial stage distribution, (2) survival and mortality trends, also in a comparison to other countries and (3) national health care costs.
Material and methods
Recommendation for abandoning testing for microscopic haematuria was issued in 1999 by the National Board of Health and Welfare of Sweden (Socialstyrelsen). We assigned the data from the time period 1997–2001 as the baseline comparator to control for delays in clinical routine changes. For analyses of initial stage distribution, the following 5-year period was used and for survival time trends, we compared the baseline to three consecutive 5-year periods, comprising a total time span of 20 years.
This study was based on open-access data in national and international cancer registries. To monitor the quality of bladder cancer care, the Swedish National Register of Urinary Bladder Cancer (SNRUBC) was initiated in 1997. The register has detailed data on 97% of bladder cancer cases diagnosed and reported to the compulsory General Cancer Registry of Sweden during the period 1997 to 2016. Information on tumour, node, metastasis (TNM) stage according to the Union for International Cancer Control (UICC) 2002 classification, grade according to the WHO (1999) and primary treatment are reported to the registry, usually by the attending urologist or nurse. Death was monitored through the Cause of Death register until 31 December 2020. In SNRUBC, survival is calculated as relative. It is the observed survival in a patient group compared to the expected in the background population with the same age and gender distribution and observed during the same calendar time and thus represents cancer survival in the absence of other causes of death
Results from the Nordic countries were obtained from NORDCAN, a database of cancer statistics for the Nordic countries. National-level aggregated data were obtained from the PC version of the NordCan database (https://nordcan.iarc.fr/en/dataviz). It includes data from Denmark, Finland, Norway, Sweden, Iceland, the Faroe Islands and Greenland (the latter three not included in this analysis). NORDCAN is a publicly available, annually updated database that provides annual counts of cancer incident cases, deaths and corresponding population size. Nordic cancer registries use each country’s personal identification number systems, enabling complete, population-based registry data linked across various local registries. The Nordic standard population was used for age-standardised rates.
The calculation of the cost of the investigations was based on a study from Liedberg et al from 2016 [7]. Data on resource use for outpatient care (including primary care), over the period from first visit to diagnosis were obtained from patient charts at primary health-care centres and hospitals. Unit costs of outpatient care, inpatient care and medications were acquired from regional and national price lists in Sweden according to regional and national price lists for 2015 (http://www.skane.se/upload/webbplatser/sodra%20regionvardsnamnden/prislista/2015/helaprislistan2015.pdf; http://www.fass.se; http://webbutik.skl.se/bilder/artiklar/pdf/7164-395-7.pdf;).
To calculate the cost for the number of patients investigated yearly in the country because of AMH before the change in policy, a study from Boman et al. from 2001 was used [8]. In their area, referrals during 1 year to a department of urology (catchment area 250,000 inhabitants) were 581. The haematuria was macroscopic in 247 cases and microscopic in 287. Of the latter 92 had concomitant symptoms leaving 195 with AMH. With a population of 10 million, this would account for 7,800 investigations yearly because of AMH in Sweden.
Statistical methods
The following methods were used in the registries. Relative survival was calculated by using the Pohar-Parmé estimate for expected survival and population mortality rates were obtained from the Human Mortality Database.
Ethics
Aggregated data from a publicly accessible database were used posing no ethical issues according to Swedish Ethical Review Authority (Dnr 2023-02814-01). We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Results
Clinical information of included patients is presented in Table 1. In total, 9,628 patients during the baseline period 1997–2001 and 10,250 during the following 5-year period 2002–2006 after the introduction of the policy. In a comparison to the baseline period, the number of patients diagnosed has increased steadily especially the low- and intermediate-risk patients (Ta G1–2). Among high-risk patients those with superficial stages, TaG3, T1 and T is remained stable while the proportion of patients diagnosed with muscle-invasive bladder cancer (T2 to T4) of all patients decreased. The detailed information on T stage is depicted for the years 1997–2006 in Figure 1 and Supplementary Table S1.
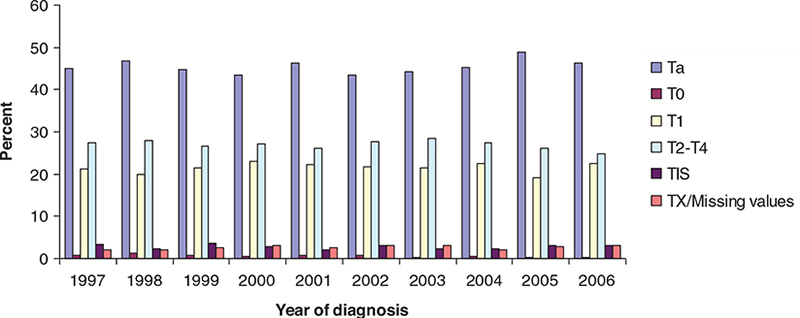
Figure 1. Distributions of initial T-stage among newly diagnosed patients with urinary bladder cancer in Sweden during 1997–2006.
The 5-year relative survival was initially unchanged but has improved significantly in the following 5-year periods (Figure 2). A similar trend was observed for overall survival. Mortality around 7 per 100,000 has remained constant during the period. In comparison to the other Nordic countries, Sweden has the highest relative survival for both genders (Table 2). It remains among those with the lowest mortality with a trend of slightly decreasing mortality during recent years (Figure 3).
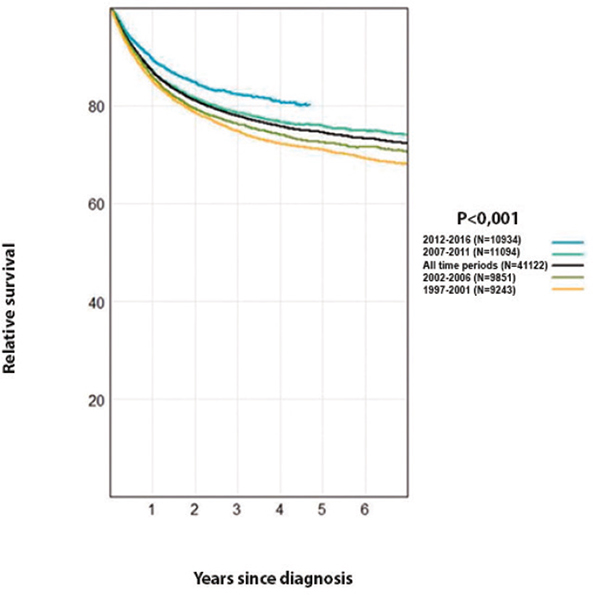
Figure 2. Relative survival analyses of patients after diagnosis at different time periods during 1997–2016 in Sweden (P value for trend).
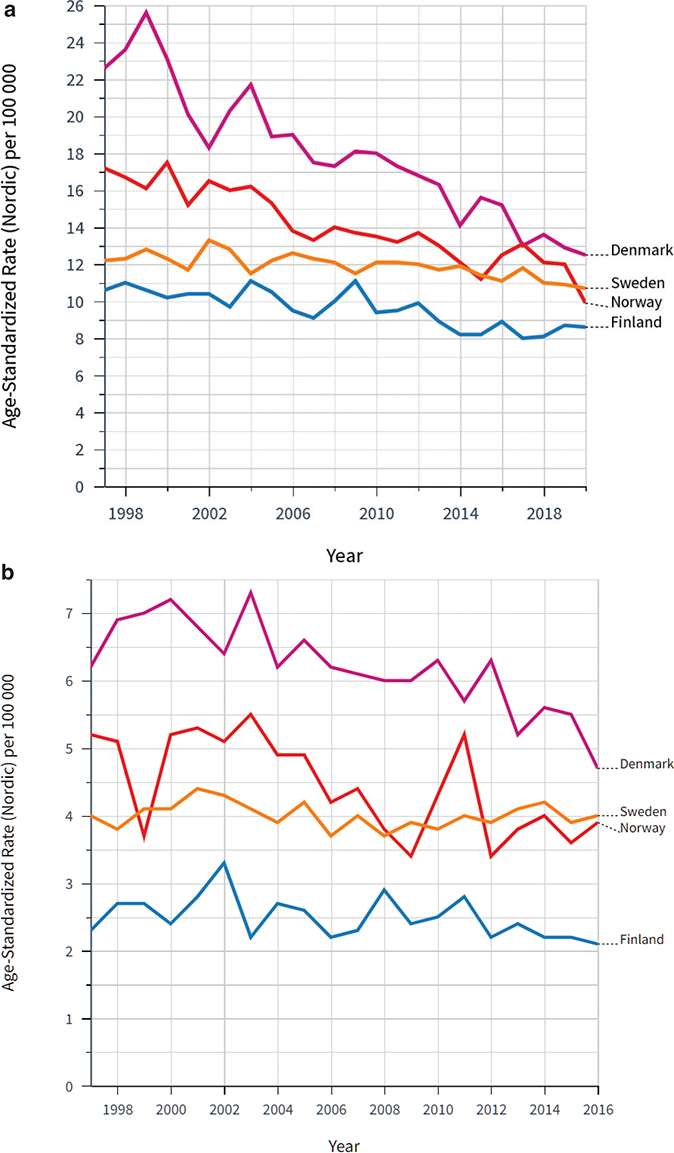
Figure 3. Age standardized mortality rate for bladder and urinary tract cancers in the Nordic countries per 100,000 (adjusted for the Nordic population) in (a) males and (b) women, 1997–2016.
The cost of cystoscopy and upper tract imaging was approximately 700 EUR per patient. Yearly cost savings because of the policy change were thus calculated to be 5.460000 EUR assuming investigations for AMH and were avoided in 7,800 patients per year. During the observed 20-year period, the total cost savings were estimated to 109,200,000 EUR.
Discussion
This follow-up provides a comprehensive overview of the outcome of a change in policy. It is based on national and international open-source registries and covers the period before and after the change in management. While there was an increasing incidence the proportion of patients diagnosed with muscle-invasive bladder cancer decreased from 27% to 22%. The 5-year relative survival has increased especially during the time from 2007. Mortality remained constant during the period. In comparison to the other Nordic countries, without policy change, Sweden has the highest relative survival and among the lowest mortality despite the difference in management. Cost savings because of the policy change were substantial. In summary, no negative effects were detected for the presentation and survival outcome of patients with bladder cancer.
As macroscopic haematuria is a key symptom to finding urothelial cancer, it would seem logical to strive for earlier detection by using dipsticks for microhaematuria. Unfortunately, this routine was introduced without proper scientific evidence. The history of testing urine for erythrocytes is not unique. It is up to this day common that new tests are introduced without evidence for clinical efficacy and properly performed cost-benefit analyses.
Most guidelines advocate that a positive test needs verification by a microscopic urinalysis, but a recent study found that both had similar accuracy [9]. It must be underscored that the subject of this follow-up is AMH. Patients with symptoms like urgency or pain should be investigated regardless of the urinary test result. With early diagnosis, tumours can be identified when smaller and easily extirpated or ablated. If abandoning the microhaematuria test led to a delayed diagnosis, this would translate to a trend towards higher tumour stages at diagnosis with a worse prognosis. No such effect was seen after the introduction of the new policy in Sweden. In fact, it was a decrease in the most lethal stages. The most feared result of a more restrictive health policy is a decrease in survival. We found the opposite, an increase in survival after the introduction of the new policy in Sweden. If that increase was less than what had been achieved with the prior, more intensive policy is a valid question. To set this in perspective, a comparison to countries with a more liberal policy could be valuable. The standards for the investigation of haematuria in the different Nordic countries were reviewed in 2019 [10]. The other countries are still investigating patients with AMH, but some have recently proposed age limits. In all countries, a similar trend in survival as in Sweden has been observed arguing against a detrimental effect of the new policy.
Unnecessary medical investigations have multiple unwanted effects. One of these is health care expenditure for common laboratory findings. The number of patients with a diagnosis of AMH in US 2012 was calculated to be almost 500,000 [11]. Regarding the cost assessment, this will of course vary between countries. A study from Canada found the cost of evaluating AMH to be $1196.85 per patient. They generally used ultrasound in this context instead of abdominal CT [12]. This cost estimate is more than double of what has been calculated with in our analysis. Besides the cost consequence of the new policy in Sweden, there was an intention to focus time and resources on bladder cancer care. Thus, in the same time period, measuring different quality indicators in this area was implemented and specifically to decrease the reported long delay in Sweden between the onset of macroscopic haematuria and diagnosis of bladder cancer [8]. Another unwanted effect is complications because of investigation procedures. The evaluation for AMH is associated with a risk of adverse events including procedural discomfort and urinary tract infection after cystoscopy, contrast-induced nephropathy and radiation exposure, all of which may impact quality of life and generate further health care costs. The increasing concern about the association between radiation-related carcinogenesis and abdominal CT has led to suggestions to discard this examination from microhaematuria investigations in the US as is common in Canada [13].
The strength of our study is that it was based on national cancer register data of high validity to which all tumours are reported in a country with a uniform national public healthcare system and virtually complete follow‐up. We assessed both mortality based on information on death certificates and relative survival, which is independent of the classification of causes of death, and the trends were consistent. Furthermore, the trends were similar to the Nordic countries, arguing against a hidden negative effect of the policy change in Sweden.
Study limitations included the retrospective nature of our review and the semi-ecological nature of the study in the sense that we do not have individual information on the exposure, that is, exactly how intense diagnostic work-up for AMH was before 1999 and after. Many urological departments returned new referrals with a comment about the new policy without registering this. We assumed that by 2002, the testing for AMH was done very seldom as it was without medico-legal consequences. Before the new policy recommendation, the health authorities had issued warnings in cases where investigations of AMH were not performed in patients later diagnosed with bladder cancer. After the new policy was recommended, similar cases were treated without leading to warnings. In adults, AMH is mainly discussed in the context of diagnosing bladder cancer. Other aetiologies are not analysed but are of minor concern for public health measures.
Smoking is the most important life style factor, and this is a potential confounder. In a global study, 13 countries, among them Norway and Sweden, recorded significant annualised rates of decline both between 1990 and 2005 and 2005 and 2015, suggesting sustained progress in tobacco control. In Denmark, there has also been a decline, but smoking is still more prevalent than in the other Nordic countries [14].
The cost assessment is intended as an estimate for illustrative purposes and reflects the situation in Sweden at a certain time period and may not precisely capture national cost savings associated with distinct diagnostic strategies, which would require more robust analysis with cost-driven data inputs. The estimation is on the cost of the investigation and does not cover possible expenditures for delayed diagnosis. Private clinics were rare in Sweden, but still the number of patients investigated in such clinics was not included and thus the cost may have been underestimated. As healthcare systems and cost levels vary for different countries, the results could therefore not be directly applied in other healthcare systems.
This analysis is based on two unique events. First the national policy change 1999 and secondly the start of the national detailed bladder cancer registry 1997, the latter making it possible to analyse possible detrimental effects of the changing management of AMH before and after the new recommendations. As it has been pointed out, there is a lack of level 1 evidence to inform guidelines for testing and diagnostic work-up for AMH. Probably this will not change in the future and thus indirect evidence is what is available as presented in this ecological study.
Conclusion
In summary, based on national and international open-source registry data the new restrictive policy was not found to adversely affect tumour presentation and survival of patients with urinary bladder cancer in Sweden. In comparison to the other Nordic countries without policy changes, Sweden remains among those with the highest survival and lowest mortality despite this restrictive policy.
References
- [1] Wallis CJD, Klaassen Z. Optimizing management of patients with microhematuria: gold down the drain? Can Urol Assoc J. 2019; 13:412–413. https://doi.org/10.5489/cuaj.6315
- [2] Rai BP, Luis Dominguez Escrig J, Vale L, et al. Systematic review of the incidence of and risk factors for urothelial cancers and renal cell carcinoma among patients with haematuria. Eur Urol. 2022; 82:182–192. https://doi.org/10.1016/j.eururo.2022.03.027
- [3] Malmström PU. Time to abandon testing for microscopic haematuria in adults? BMJ. 2003; 326:813–815. https://doi.org/10.1136/bmj.326.7393.813
- [4] Kotb AF, Attia D. High-grade microscopic hematuria in adult men can predict urothelial malignancy. Can Urol Assoc J. 2014; 8:7–8. https://doi.org/10.5489/cuaj.1746
- [5] Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int. 2018; 121:176–183. https://doi.org/10.1111/bju.14016
- [6] Barocas DA, Boorjian SA, Alvarez RD, et al. Microhematuria: AUA/SUFU guideline. J Urol. 2020; 204:778–786. https://doi.org/10.1097/JU.0000000000001297
- [7] Liedberg F, Gerdtham U, Gralén K, et al. Fast-track access to urologic care for patients with macroscopic haematuria is efficient and cost-effective: results from a prospective intervention study. Br J Cancer. 2016; 115:770–775. https://doi.org/10.1038/bjc.2016.265
- [8] Boman H, Hedelin H, Holmäng S. The results of routine evaluation of adult patients with haematuria analysed according to referral form information with 2year follow-up. Scand J Urol Nephrol. 2001; 35:497–501. https://doi.org/10.1080/003655901753367613
- [9] Matulewicz RS, DeLancey JO, Pavey E, et al. Dipstick urinalysis as a test for microhematuria and occult bladder cancer. Bladder Cancer. 2017; 3:45–49. https://doi.org/10.3233/BLC-160068
- [10] Malmström PU, Skaaheim Haug E, Boström PJ, et al. Progress towards a Nordic standard for the investigation of hematuria: 2019. Scand J Urol. 2019; 53: 1–6. https://doi.org/10.1080/21681805.2018.1555187
- [11] Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Int Med. 2017; 177:800–807. https://doi.org/10.1001/jamainternmed.2017.0739
- [12] Assmus MA, Beyer DB, Hanks J, et al. Quality and cost assessment of Canadian Urological Association microscopic hematuria guidelines in clinical practice: turning urine into gold. Can Urol Assoc J. 2019; 13:406–411. https://doi.org/10.5489/cuaj.5809
- [13] Waisbrod S, Natsos A, Wettstein MS, et al. Assessment of diagnostic yield of cystoscopy and computed tomographic urography for urinary tract cancers in patients evaluated for microhematuria: a systematic review and meta-analysis. JAMA Netw Open. 2021; 4:e218409. https://doi.org/10.1001/jamanetworkopen.2021.8409
- [14] GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017; 389:1885–1906.