ORIGINAL RESEARCH ARTICLE
Predictors for complication in renal cancer surgery: a national register study
John Åkerlunda,b, Pernilla Sundqvistc, Börje Ljungbergd, Sven Lundstama,b,e, Ralph Peekera,b, Marianne Månssona and Anna Grenabo Bergdahla,b
aDepartment of Urology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bRegion Västra Götaland, Department of Urology, Sahlgrenska University Hospital, Gothenburg, Sweden; cDepartment of Urology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden; dDepartment of Surgical and Perioperative Sciences, Umeå University, Umeå, Sweden; eDepartment of Oncology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
ABSTRACT
Objective: Nationwide register data provide unique opportunities for real-world assessment of complications from different surgical methods. This study aimed to assess incidence of, and predictors for, post-operative complications and to evaluate 90-day mortality following different surgical procedures and thermal ablation for renal cell carcinoma (RCC).
Material and methods: All patients undergoing surgical treatment and thermal ablation for RCC in Sweden during 2015–2019 were identified from the National Swedish Kidney Cancer Register. Frequencies and types of post-operative complications were analysed. Logistic regression models were used to identify predictors for 90-day major (Clavien-Dindo grades III–V) complications, including death.
Results: The overall complication rate was 24% (1295/5505), of which 495 (8.7%) were major complications. Most complications occurred following open surgery, of which bleeding and infection were the most common. Twice as many complications were observed in patients undergoing open surgery compared to minimally invasive surgery (20% vs. 10%, P < 0.001). Statistically significant predictors for major complications irrespective of surgical category and technique were American society of anesthiologists (ASA) score, tumour diameter and serum creatinine. Separating radical and partial nephrectomy, surgical technique remained a significant risk factor for major complications. Most complications occurred within the first 20 days. The overall 90-day readmission rate was 6.2%, and 30- and 90-day mortality rates were 0.47% and 1.5%, respectively.
Conclusions: In conclusion, bleeding and infection were the most common major complications after RCC surgery. Twice as many patients undergoing open surgery suffer a major post-operative complication as compared to patients subjected to minimally invasive surgery. General predictors for major complications were ASA score, tumour size, kidney function and surgical technique.
KEYWORDS: Renal cell carcinoma; surgery; complications; mortality; register
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 38–45. https://doi.org/10.2340/sju.v58.12356.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 12 May 2023; Accepted: 27 June 2023; Published: 21 August 2023
CONTACT John Åkerlund john.akerlund@me.com Sahlgrenska University Hospital, Bruna stråket 11, SE-405 83 Gothenburg, Sweden
Competing interests and funding: No potential conflict of interest was reported by the author(s).
Introduction
Treatment of renal cell carcinoma (RCC) has evolved rapidly during recent years, both for localized and metastatic diseases. Emerging technologies have been adopted with enthusiasm, and traditional open RCC surgery has in many cases been replaced by minimally invasive options. Today, there are four major management alternatives for localized RCC: radical nephrectomy, partial nephrectomy, thermal ablation and active surveillance [1]. Several treatment strategies may exist for the same type of tumour, and hence, patient selection is crucial [2–4]. Balancing the competing risks related to age, comorbidities and performance status is a difficult task; many patients and tumours fall into a grey area. Studies in the past have tried to develop adjusted treatment models for selected tumour and patient groups [5]. Nationwide real-world data offer opportunities to monitor frequency, grade and type of complications with different treatments and allow for an improved transparency of the healthcare system, which in turn can aid in patient counselling. Post-operative complications are still the most commonly used method for assessing quality of surgery [6]. Although several reports have demonstrated low morbidity and mortality rates associated with RCC surgery [7–11], these reports frequently only evaluate one treatment method or one certain tumour stage specifically [12–15]. Hitherto, there is no nationwide population-based study published, which evaluate complications of all available RCC treatment methods.
Our primary aim was to analyse risk factors associated with major post-operative complications, including death after RCC surgery, and compare these in relation to open/minimally invasive surgery and radical nephrectomy/partial nephrectomy.
Material and methods
Data collection
In 2005, devoted RCC experts in Sweden started a kidney cancer quality register, the National Swedish Kidney Cancer Register. The purpose was to include all patients with RCC in Sweden and monitor treatment outcome [10, 16]. In 2015, post-operative complications were added to the National Swedish Kidney Cancer Register; moreover, it is validated and captures 99% of all patients with RCC registered in the Swedish Cancer Registry, to which registration is mandated by law [17]. Hence, the National Swedish Kidney Cancer Register contains data from all Swedish healthcare regions and hospitals, no matter the size, as described by Thorstenson et al. [10]. The reported data are registered by either treating surgeons or designated staff. Before the final registration is made to the register, a person within each Regional Cancer Center declares that all data have been included; this person has not been involved in the treatment. Ethical approval was obtained from the Regional Ethics Committee in northern Sweden (Dnr 2012-418-31M and Dnr 2014-301-32M).
Study design and patient population
For the present study, data on all surgical RCC treatments (open and minimally invasive radical nephrectomy and open and minimally invasive partial nephrectomy, and thermal ablation, herein viewed as a surgical method), prospectively registered in the National Swedish Kidney Cancer Register between 2015 and 2019, were extracted. The study population consisted of 5489 patients who underwent 5619 treatments. Amongst those, 114 treatments in 40 patients were excluded from further analyses (patients < 18 years, patients managed expectantly, patients with RCC in transplant kidneys and patients with misregistrations), leaving 5505 treatments in 5449 patients for analyses (Figure S1).
Demographics included age, gender, body mass index (BMI), European Cooperative Oncology Group performance status (ECOG), ASA score, pre- and post-operative eGFR and RENAL-score. Tumour characteristics included tumour diameter, M-stage and N-stage at diagnosis, histopathology and pT-stage. Perioperative characteristics included surgical technique, operation time and estimated intraoperative blood loss. Minimally invasive techniques included traditional laparoscopy and robotic-assisted laparoscopy. Post-operative parameters were length of stay, unplanned readmissions and complications divided into ‘surgical’ and ‘general’ and graded according to the standardized Clavien-Dindo system (complication grades I–II considered ‘minor’ and grades III–V considered ‘major’) [6, 18]. Data on overall survival (OS) within 90 days were collected from the population register.
Statistical analysis
Descriptive statistics of patient characteristics were presented with medians and interquartile ranges (IQR) for continuous variables and frequencies and proportions for categorical variables. Demographics and tumour characteristics were stratified and compared between surgical type radical nephrectomy/partial nephrectomy and open/minimally invasive technique. The Mann–Whitney U test and Fisher’s exact test were used to compare continuous and categorical variables, respectively.
We used multivariable logistic regression models to estimate odds ratios (OR) with 95% confidence intervals (CIs) for potential risk factors for major complications and deaths within 90 days. Factors explored were ‘patient- and tumour related’ (gender, age, BMI, ASA score, tumour diameter and serum creatinine) and ‘surgery related’ (surgical category and surgical technique). Serum creatinine, tumour diameter and operative time were skewedly distributed, and, hence, to fulfil model requirements, they were logarithmically transformed on a base 2 scale; hence, the interpretation of the ORs for these variables regards doubling rather than one unit change. ASA score was categorized into ASA 1–2 and 3–4. Estimated blood loss was categorized into 0–199 mL, 200–499 mL and >500 mL.
In the multivariable analysis, patient and tumour factors were initially analysed on all data and thereafter in subgroups (radical nephrectomy/partial nephrectomy), where also surgical technique was included. Operation time and estimated blood loss were also added in the subgroup analyses. Treatments with missing data on one or more potential predictors were excluded (N = 86). Cumulative risk of major complications and death within 90 days for all surgical techniques were calculated as one minus the Kaplan–Meier estimates. Stacked cumulative incidence curves of deaths and major complications were presented. Thermal ablation was included in tables but excluded from the analyses due to too few treatments. A P-value of ≤ 0.05 was considered to be statistically significant. Statistical analyses were carried out using IBM SPSS version 27 and R Statistical Software (Version 4.0.4).
Results
Patients and treatments
Descriptive characteristics are shown in Tables 1 and 2. The most common surgical treatment was open radical nephrectomy (32%), followed by minimally invasive radical nephrectomy (21%), minimally invasive partial nephrectomy (19%), open partial nephrectomy (19%) and thermal ablation (9.3%). The most advanced tumour stages were found amongst patients undergoing radical nephrectomy; 20% and 6.0% had M1 disease and 54% and 28% were ≥ pT3 amongst open radical nephrectomy and minimally invasive radical nephrectomy, respectively (Table 1). Furthermore, patients selected for radical nephrectomy were older and had more comorbidities than those selected for partial nephrectomy.
| Surgical technique | Radical nephrectomy | Partial nephrectomy | Thermal ablation | ||
| Open | Minimally invasive | Open | Minimally invasive | Percutaneous | |
| Treatments, no. (%) | 1745 (31.7) | 1141 (20.7) | 1049 (19.1) | 1056 (19.2) | 514 (9.3) |
| Tumour histology (%) | |||||
| Clear cell | 1397 (80.1) | 904 (79.3) | 705 (67.2) | 699 (66.2) | 321 (62.4) |
| Papillary | 169 (9.7) | 130 (11.4) | 199 (19.0) | 213 (20.2) | 116 (22.5) |
| Chromophobe | 116 (6.6) | 81 (7.1) | 98 (9.3) | 105 (9.9) | 43 (8.4) |
| Collecting duct | 8 (0.5) | 5 (0.4) | 1 (0.1) | 0 (0.0) | 1 (0.2) |
| Unclassified renal cell carcinoma | 55 (3.1) | 21 (1.8) | 46 (4.4) | 39 (3.7) | 33 (6.5) |
| Post-operative eGFR (mL/min/1.73 m2) [IQR] | 48 [37–59] | 47 [36–58] | 66 [53–78] | 71 [59–82] | 60 [48–72] |
| Post-operative, serum creatinine (µmol/L) [IQR] | 117 [97–140] | 117 [99–139] | 87 [75–107] | 83 [71–97] | 90 [75–111] |
| Missing data | 53 (3.0) | 37 (3.2) | 33 (3.1) | 40 (3.8) | 72 (14.0) |
| Operation time (min) [IQR] | 154 [120–205] | 156 [125–195] | 150 [120–192] | 170 [135–203] | 13 [6–45] |
| Missing data | 12 (0.7) | 5 (0.4) | 5 (0.5) | 7 (0.7) | 43 (8.4) |
| Estimated blood loss (mL) [IQR] | 500 [200–1100] | 100 [25–200] | 400 [200–700] | 100 [50–250] | NR |
| Missing data | 8 (0.5) | 3 (0.3) | 5 (0.5) | 2 (0.2) | 0 |
| Length of stay (days) [IQR] | 6 [5–8] | 3 [2–4] | 6 [5–7] | 3 [2–4] | 1 [1–1] |
| Missing data | 2 (0.1) | 0 | 0 | 0 | 0 |
| Unplanned readmissiona (%) | |||||
| Yes | 196 (11.3) | 93 (8.2) | 96 (9.2) | 91 (8.7) | 42 (8.2) |
| Clavien-Dindo, complications (grade) (%) | |||||
| I–II | 417 (23.9) | 108 (9.5) | 268 (25.5) | 159 (15.1) | 51 (9.9) |
| III–V | 210 (12.0) | 58 (5.1) | 131 (12.5) | 66 (6.2) | 14 (2.7) |
| Data are shown as median [interquartile range] and frequencies (percentages). NR (not registered). Percentages are calculated after excluding missing data. | |||||
| a Readmission within 90 days of surgery. | |||||
| eGFR: estimated glomerular filtration rate; IQR: interquartile range. | |||||
Risk of complications
In total, there were 1814 complications of any grade within 90 days (Table 3). Of the 1126 surgical complications, 483 (43%) were major, and of the 688 general complications, 99 (14%) were major. The overall 1814 complications were distributed in 1295 out of the 5505 treatments (24%), of which 479 (37%) had major complications. No significant difference was observed regarding surgical complications in radical nephrectomy and partial nephrectomy (Table SIa).
Major complications occurred more often after open surgery irrespective of category (radical nephrectomy/partial nephrectomy): 210 (12%) open radical nephrectomy, 58 (5.1%) minimally invasive radical nephrectomy, 131 (13%) open partial nephrectomy, 66 (6.2%) minimally invasive partial nephrectomy and 14 thermal ablation (2.7%) (Table 1). Rates of complications in relation to surgical category and technique are shown in Table SI. A doubled risk of complications was observed in patients undergoing open surgery when compared to minimally invasive surgery (20% vs. 10%, P < 0.001). When adjusting for gender, age, BMI, ASA score, tumour diameter, serum creatinine and surgical technique, open surgery implied an approximately two-fold increased risk of major complications compared with minimally invasive surgery, irrespective of surgical category (radical nephrectomy/partial nephrectomy) 11% (95% CI: 9–13) versus 6% (95% CI: 4–8) in open radical nephrectomy and minimally invasive radical nephrectomy, respectively, and 12% (95% CI: 9–14) versus 7% (95% CI: 5–9) in open partial nephrectomy and minimally invasive partial nephrectomy, respectively.
Predictors for complications
In a model with patient and tumour factors as potential predictors of major complications, irrespective of surgical category and technique, significant predictors were ASA score (OR: 1.90; 95% CI: 1.54–2.34, P < 0.001), tumour diameter (OR: 1.30; 95% CI: 1.17–1.46, P < 0.001) and serum creatinine (OR: 1.28; 95% CI: 1.07–1.54, P = 0.009) (Table SII). Table SIII shows subgroup analysis divided into radical nephrectomy and partial nephrectomy with the potential predictors of gender, age, BMI, ASA score, tumour diameter, serum creatinine and surgical technique. In the partial nephrectomy group, significant risk factors for major complications were open surgical technique (OR: 1.77; 95% CI: 1.27–2.47, P < 0.001) and large tumour diameter (OR: 1.47; 95% CI: 1.15–1.88, P = 0.002). In radical nephrectomy, significant risk factors of major complications were higher ASA score (OR: 2.37; 95% CI: 1.80–3.12, P < 0.001), open technique (OR: 2.10; 95% CI: 1.53–2.93, P < 0.001), large tumour diameter (OR: 1.40; 95% CI: 1.14–1.72, P < 0.001) and higher serum creatinine (OR: 1.35; 95% CI: 1.08–1.68, P = 0.010).
Regarding ‘perioperative factors’, the median estimated blood loss was lower, and the operation time was slightly longer in minimally invasive surgery as compared to open surgery (Table 2). However, even after adjusting for operation time and estimated blood loss as independent risk factors for major complications, in addition to patient and tumour factors, surgical technique (open vs. minimally invasive) remained a significant predictor for complications both in radical nephrectomy (OR: 1.63; 95% CI: 1.13–2.36, P = 0.008) and partial nephrectomy (OR: 1.62; 95% CI: 1.12–2.34, P = 0.01), although with a lower OR. Furthermore, ASA and serum creatinine remained significant in the radical nephrectomy subgroup analysis, whilst in the partial nephrectomy subgroup analysis, tumour diameter and BMI were significant. For both radical nephrectomy and partial nephrectomy, estimated blood loss and operation time were significant risk factors (Table SV).
Unplanned readmissions
In total, 518 patients were readmitted within 90 days of surgery, of which 340 were readmitted within 30 days. Readmissions most frequently occurred after open radical nephrectomy (Table 2). The most common causes for 90-day readmission were infection, abdominal/flank pain and bleeding (data not shown).
Survival
The overall mortality rate within 30 and 90 days of surgery was 0.47% (26/5505) and 1.5% (83/5505), respectively. In a logistic regression model for overall mortality within 90 days, only ASA score, tumour diameter and surgical technique were statistically significant (Table SIV).
Figure 1 shows the cumulative incidence of having Clavien-Dindo III–IV complications and/or death within 90 days, divided by surgical type and technique. Furthermore, these patients are divided into three groups: Clavien-Dindo III–IV & no death, Clavien-Dindo III–IV & death and no Clavien-Dindo III–IV & death. Patients treated with open radical nephrectomy had the highest risk of death within 90 days (3.3%), followed by minimally invasive radical nephrectomy (0.9%).
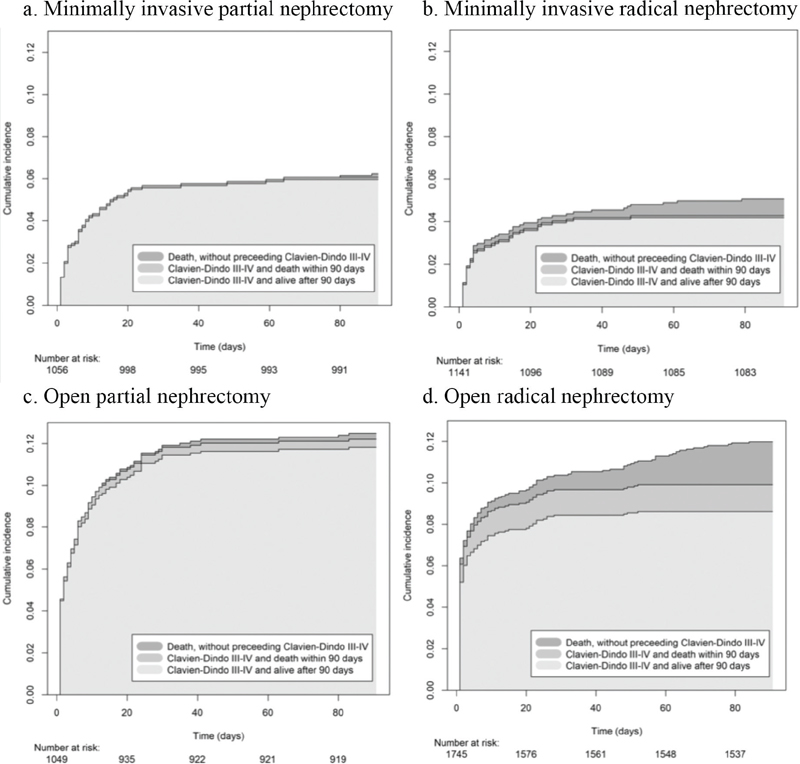
Figure 1. Stacked cumulative incidence of Clavien-Dindo III–IV complications and/or death within 90 days, divided by surgical type and technique.
Discussion
In this large register-based study, bleeding and infections were the most common major complications after surgery for renal cancer. Patients who underwent open surgery were twice as likely to experience complications as those who underwent minimally invasive surgery. Other parameters predictive for major complications were patient performance and tumour size.
A better understanding of the nature of surgical complications is important to improve the overall quality of care. Although mortality rates are low following RCC surgery, major post-operative complications are observed in 2.7%–13%, and readmission rates range from 8.2% to 11% depending on surgical category and technique [5, 13, 19]. When striving towards the safest possible surgical practice, quality indicators can be useful to minimize quality variations in RCC care [5, 20, 21], several recent studies have compared perioperative morbidity and mortality associated with different surgical treatments for RCC [12, 22–24]. Our study adds important value to existing knowledge on the risks with different surgical treatments for RCC.
Of the 5505 surgical treatments for RCC between 2015 and 2019 in Sweden, open radical nephrectomy was the most common procedure, with 1745 treatments. The fact that open radical nephrectomy was the more used treatment method despite a large proportion (24%) of T1 tumours (≤ 7 cm) can probably be explained by the fact that robotic surgery had not been fully implemented in Sweden at the time. The adoption of nephron-sparing surgery was also gradual rather than immediate after the EAU and AUA guidelines in 2009 recommended partial nephrectomy as the preferred treatment option for T1 tumours. When observing treatment trends in the National Swedish Kidney Cancer Register, the use of minimally invasive techniques has gradually increased, and since 2018, the majority of treatments for RCC are performed minimally invasively [16].
Most complications following renal cancer surgery are related to bleeding and infections (Table 3). The rate of treatments with surgical and general complications following RCC surgery was 16% and 10%, respectively. Our study shows, in line with a previous report, that most major complications in both radical nephrectomy/partial nephrectomy appeared within the first 20 days [24]. The open procedures had a higher rate of post-operative complications compared to minimally invasive, roughly 25% versus 12% for Clavien-Dindo I–II and 10% versus 6% for Clavien-Dindo III–V, confirming previous studies [19, 25, 26]. Furthermore, previous studies have shown a larger amount of bleeding and longer hospital stay in the open surgery group, tallying with the data obtained in the present study [27]. However, the large difference observed between open and minimally invasive procedures can presumably not solely be explained by the technique itself. There is, no doubt, a selection bias in the open group compared to the minimally invasive; healthier patients and smaller and less advanced tumours were treated with a minimally invasive technique. Furthermore, there are factors lacking in our data, which possibly could explain the difference in a better way, such as prior abdominal surgery, locally advanced tumours invading the caval vein and adjacent organs and degree of vascular and nodal involvement [28]. In a similar vein, increasing comorbidity, higher pre-operative serum creatinine and larger tumours have been reported to increase the risk of complications [5, 29].
We further opted to see if the difference in complications between open/minimally invasive techniques could be explained by a higher estimated blood loss and longer operation time. When we added operation time and bleeding in the models, they became significant in both radical nephrectomy and partial nephrectomy, but surgical technique remained significant with ORs approximately 1.6. Therefore, it is hard to argue that operation time and estimated blood loss solely explain the difference between the techniques. These measures can be seen as indicators of intraoperative complexity rather than apparent risk factors known in advance. Except a higher intraoperative risk, Rouseel et al. showed that patients with metastatic burden, treated with adjacent organ removal and/or thrombectomy, were more associated with major complications [30].
There is a close relationship between rate and severity of complications and OS. Reduction of post-operative complications is an independent predictor of both short- and long-term OS for patients with RCC [8]. Patients undergoing radical nephrectomy had considerably higher overall mortality than patients undergoing partial nephrectomy, and within these groups, the rates were highest with open surgery. Beyond surgical technique, tumour size and ASA score were significant predictors of overall mortality within 90 days, in accordance with previous reports [5, 29, 31]. Prior studies have, moreover, shown a safe implementation of partial nephrectomy regardless of technique, showing preserved oncologic control, better renal preservation and similar or better cancer specific mortality and all-cause mortality [13, 22, 23, 29, 31, 32].
Our study appears to confirm previous studies that demonstrate advantages of minimally invasive techniques with regard to decreased length of stay, readmission rates, complication rates and 90-day mortality compared to open surgery [13, 27, 31].
It is reasonable to believe that with the ongoing trend towards more minimally invasive surgery, as well as the stage migration that has been observed during recent years [33], a decreased complication rate can be expected in the future.
Important limitations of the present study were the inability to control for socioeconomic status, prior surgery, surgeon experience, and hospital and surgeon volume, which all may have affected the outcome. With register-based data collected in all the regions and hospitals in Sweden, discrepancies may be possible due to uniformity in collecting data and may have resulted in coding discrepancies, as mentioned by Thorstenson et al. [10]. Furthermore, to accurately compare specific treatment methods and analyse post-operative complications, a randomized set-up would be needed, with strict inclusion criteria and treatment indications. However, as prospective RCTs comparing surgical groups or techniques have generally been difficult to conduct, population-based register studies are valid alternatives. The present study is the first prospective study describing post-operative complications of all types of surgery and thermal ablation for RCC, compiling unselected real-world data from a nationwide population-based registry; moreover, it contains virtually all RCC treatments in Sweden, already proved to have a high completeness and validity [17].
Conclusion
In conclusion, bleeding and infection were the most common major complications after RCC surgery. Twice as many patients who underwent open surgery experienced a major post-operative complication as compared to patients subjected to minimally invasive surgery. General predictors for major complications were ASA score, tumour size, kidney function and surgical technique. These findings, based on a real-world data, can be used for patient counselling, and as a basis for further investigations of complications after RCC treatments.
Acknowledgements
Thanks to the members of the National Swedish Kidney Cancer Register steering committee and collaborators at the Regional Cancer Centre, Stockholm, for providing data from the register. This work was supported by funds from Märta and Gustaf Ågren’s research foundation and from Anna-Lisa and Bror Björnsson’s research foundation.
ORCID
John Åkerlund  http://orcid.org/0000-0002-0197-9009
http://orcid.org/0000-0002-0197-9009
References
- [1] Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399-410. https://doi.org/10.1016/j.eururo.2022.03.006
- [2] Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J Urol. 2021;206(2):199–208. https://doi.org/10.1097/JU.0000000000001911
- [3] Campbell SC, Uzzo RG, Karam JA, Chang SS, Clark PE, Souter L. renal mass and localized renal cancer: evaluation, management, and follow-up: AUA Guideline: part II. J Urol. 2021;206(2):209–18. https://doi.org/10.1097/JU.0000000000001912
- [4] Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2019 Update. Eur Urol. 2019;75(5):799–810. https://doi.org/10.1016/j.eururo.2019.02.011
- [5] Psutka SP, Gulati R, Jewett MAS, Fadaak K, Finelli A, Legere L, et al. A clinical decision aid to support personalized treatment selection for patients with clinical T1 renal masses: results from a multi-institutional competing-risks analysis. Eur Urol. 2022;81:576–85. https://doi.org/10.1016/j.eururo.2021.11.002
- [6] Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012;61(2):341–9. https://doi.org/10.1016/j.eururo.2011.10.033
- [7] Stang A, Büchel C. Renal surgery for kidney cancer in Germany 2005–2006: length of stay, risk of postoperative complications and in-hospital death. BMC Urol. 2014;14:74. https://doi.org/10.1186/1471-2490-14-74
- [8] Tan HJ, Hafez KS, Ye Z, Wei JT, Miller DC. Postoperative complications and long-term survival among patients treated surgically for renal cell carcinoma. J Urol. 2012;187(1):60–6. https://doi.org/10.1016/j.juro.2011.09.031
- [9] Kim SP, Leibovich BC, Shah ND, Weight CJ, Borah BJ, Han LC, et al. The relationship of postoperative complications with in-hospital outcomes and costs after renal surgery for kidney cancer. BJU Int. 2013;111(4):580–8. https://doi.org/10.1111/j.1464-410X.2012.11122.x
- [10] Thorstenson A, Bergman M, Scherman-Plogell AH, Hosseinnia S, Ljungberg B, Adolfsson J, et al. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005–2010: a population-based study from the national Swedish kidney cancer register. Scand J Urol. 2014;48(3):231–8. https://doi.org/10.3109/21681805.2013.864698
- [11] Hjelle KM, Johannesen TB, Beisland C. Postoperative 30-day mortality rates for kidney cancer are dependent on hospital surgical volume: results from a Norwegian population-based study. Eur Urol Focus. 2017;3(2–3):300–7. https://doi.org/10.1016/j.euf.2016.10.001
- [12] Gershman B, Bukavina L, Chen Z, Konety B, Schumache F, Li L, et al. The association of robot-assisted versus pure laparoscopic radical nephrectomy with perioperative outcomes and hospital costs. Eur Urol Focus. 2020;6(2):305–12. https://doi.org/10.1016/j.euf.2018.10.004
- [13] Venkatramani V, Koru-Sengul T, Miao F, Nahar B, Prakash NS, Swain S, et al. A comparison of overall survival and perioperative outcomes between partial and radical nephrectomy for cT1b and cT2 renal cell carcinoma – analysis of a national cancer registry. Urol Oncol. 2018;36(3):90.e9–14. https://doi.org/10.1016/j.urolonc.2017.11.008
- [14] Mari A, Antonelli A, Bertolo R, Bianchi G, Borghesi M, Ficarra V, et al. Predictive factors of overall and major postoperative complications after partial nephrectomy: results from a multicenter prospective study (The RECORd 1 project). Eur J Surg Oncol. 2017;43(4):823–30. https://doi.org/10.1016/j.ejso.2016.10.016
- [15] Larcher A, Fossati N, Tian Z, Boehm K, Meskawi M, Valdivieso R, et al. Prediction of complications following partial nephrectomy: implications for ablative techniques candidates. Eur Urol. 2016;69(4): 676–82. https://doi.org/10.1016/j.eururo.2015.07.003
- [16] National Swedish Kidney Cancer Register, National Report, 2021. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/urinvagar/njurcancer/kvalitetsregister/ [cited 15 March 2023].
- [17] Landberg A, Bruce D, Lindblad P, Ljungberg B, Lundstam S, Thorstenson A, et al. Validation of data quality in the National Swedish Kidney Cancer Register. Scand J Urol. 2021;55(2):142–8. https://doi.org/10.1080/21681805.2021.1885485
- [18] Yoon PD, Chalasani V, Woo HH. Use of Clavien-Dindo classification in reporting and grading complications after urological surgical procedures: analysis of 2010 to 2012. J Urol. 2013;190(4):1271–4. https://doi.org/10.1016/j.juro.2013.04.025
- [19] Garisto J, Bertolo R, Dagenais J, Sagalovich D, Fareed K, Fergany A, et al. Robotic versus open partial nephrectomy for highly complex renal masses: comparison of perioperative, functional, and oncological outcomes. Urol Oncol. 2018;36(10):471.e1–9. https://doi.org/10.1016/j.urolonc.2018.06.012
- [20] Lawson KA, Saarela O, Abouassaly R, Kim SP, Breau RH, Finelli A. The impact of quality variations on patients undergoing surgery for renal cell carcinoma: a National Cancer Database Study. Eur Urol. 2017;72(3):379–86. https://doi.org/10.1016/j.eururo.2017.04.033
- [21] Rossi SH, Klatte T, Stewart GD. Quality of life outcomes in patients with localised renal cancer: a literature review. World J Urol. 2018;36(12):1961–72. https://doi.org/10.1007/s00345-018-2415-3
- [22] Buffi NM, Saita A, Lughezzani G, Porter J, Dell’Oglio P, Amparore D, et al. Robot-assisted Partial Nephrectomy for Complex (PADUA Score ≥10) tumors: techniques and results from a multicenter experience at four high-volume centers. Eur Urol. 2020;77(1):95–100. https://doi.org/10.1016/j.eururo.2019.03.006
- [23] Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: a multicenter analysis (ROSULA Collaborative Group). Eur Urol. 2018;74(2):226–32. https://doi.org/10.1016/j.eururo.2018.05.004
- [24] Sood A, Abdollah F, Sammon JD, Kapoor V, Rogers CG, Jeong W, et al. An evaluation of the timing of surgical complications following nephrectomy: data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). World J Urol. 2015;33(12):2031–8. https://doi.org/10.1007/s00345-015-1564-x
- [25] Kaisa E, Veitonmäki T, Ettala O, Ronkainen H, Isotalo T, Nykopp T, et al. Does every Clavien-Dindo complication matter? A national multi-center study in kidney cancer surgery. Scand J Urol. 2021;55:441–7.
- [26] Hadjipavlou M, Khan F, Fowler S, Joyce A, Keeley FX, Sriprasad S. Partial vs radical nephrectomy for T1 renal tumours: an analysis from the British Association of Urological Surgeons Nephrectomy Audit. BJU Int. 2016;117(1):62–71. https://doi.org/10.1111/bju.13114
- [27] Liu JJ, Leppert JT, Maxwell BG, Panousis P, Chung BI. Trends and perioperative outcomes for laparoscopic and robotic nephrectomy using the National Surgical Quality Improvement Program (NSQIP) database. Urol Oncol. 2014;32(4):473–9. https://doi.org/10.1016/j.urolonc.2013.09.012
- [28] Abel EJ, Spiess PE, Margulis V, Master VA, Mann M, Zargar-Shoshtari K, et al. Cytoreductive nephrectomy for renal cell carcinoma with venous tumor thrombus. J Urol. 2017;198(2):281–8. https://doi.org/10.1016/j.juro.2017.03.011
- [29] Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71(4):606–17. https://doi.org/10.1016/j.eururo.2016.08.060
- [30] Roussel E, Campi R, Larcher A, Verbiest A, Antonelli A, Palumbo C, et al. Rates and predictors of perioperative complications in cytoreductive nephrectomy: analysis of the registry for metastatic renal cell carcinoma. Eur Urol Oncol. 2020;3(4):523–9. https://doi.org/10.1016/j.euo.2020.04.006
- [31] Dursun F, Elshabrawy A, Wang H, Rodriguez R, Liss MA, Kaushik D, et al. Survival after minimally invasive vs. open radical nephrectomy for stage I and II renal cell carcinoma. Int J Clin Oncol. 2022;27(6):1068–76. https://doi.org/10.1007/s10147-022-02153-5
- [32] Bradshaw AW, Autorino R, Simone G, Yang B, Uzzo RG, Porpiglia F, et al. Robotic partial nephrectomy vs minimally invasive radical nephrectomy for clinical T2a renal mass: a propensity score-matched comparison from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. BJU Int. 2020;126(1):114–23. https://doi.org/10.1111/bju.15064
- [33] Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529–42. https://doi.org/10.1016/j.eururo.2022.08.019