ORIGINAL RESEARCH ARTICLE
Diagnostic pathways and treatment strategies in upper tract urothelial carcinoma in Sweden between 2015 and 2021: a population-based survey
Fredrik Liedberga,b , Oskar Hagbergb, Firas Aljaberyc, Truls Gårdmarkd
, Oskar Hagbergb, Firas Aljaberyc, Truls Gårdmarkd , Staffan Jahnsonc
, Staffan Jahnsonc , Tomas Jerlströme, Viveka Ströckf
, Tomas Jerlströme, Viveka Ströckf , Karin Söderkvistg
, Karin Söderkvistg , Anders Ullénh, Johannes Bobjera,b
, Anders Ullénh, Johannes Bobjera,b
aDepartment of Urology Skåne University Hospital, Malmö, Sweden; bInstitution of Translational Medicine, Lund University, Malmö, Sweden; cDepartment of Clinical and Experimental Medicine, Division of Urology, Linköping University, Linköping, Sweden; dDepartment of Clinical Sciences, Danderyd Hospital, Karolinska Institute, Stockholm, Sweden; eDepartment of Urology, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden; fDepartment of Urology, Sahlgrenska University Hospital and Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; gDepartment of Radiation Sciencies, Umeå University, Umeå, Sweden; hDepartment of Oncology-Pathology, Karolinska Institutet, Stockholm, Sweden; Department of Pelvic Cancer, Genitourinary Oncology and Urology unit, Karolinska University Hospital, Stockholm, Sweden
ABSTRACT
Objective: To report national data on diagnostics and treatment of upper tract urothelial carcinoma (UTUC) from the Swedish National Registry of Urinary Bladder Cancer (SNRUBC).
Patients and methods: Data from 2015 to 2021 were retrieved, and descriptive analyses were performed regarding incidence, diagnostic modalities, preoperative tumor staging, quality indicators for treatment including the use of standardized care pathways (SCP) and multidisciplinary tumor boards (MDTB). Time trends were explored for the study period.
Results: Registrations included 1,213 patients with renal pelvic cancer and 911 patients with ureteric cancer with a median age of 74 (interquartile range [IQR] 70–77) and 75 (IQR 71–78) years, respectively. Incidence rates of UTUC were stable, as were proportions of curative treatment intent. Median number of days from referral to treatment was 76 (IQR 57–99) and 90 (IQR 72–118) days, respectively, for tumors of the renal pelvis and ureter, which remained unchanged after introduction of SCP in 2016. Noticeable trends included stable use of kidney-sparing surgery and increased use of MDTB. For radical nephroureterectomy (RNU), robot-assisted technique usage increased even for non-organ-confined tumors (cT3-4) and in one out of three patients undergoing RNU a bladder cuff excision was not registered.
Conclusions: The population-based SNRUBC with high coverage contributes to the knowledge about UTUC with granular and generalizable data. The present study reveals a high proportion of patients not subjected to curatively intended treatment and suggests unmet needs to shorten lead times to treatment and use of bladder cuff excision when performing radical surgery for UTUC in Sweden.
KEYWORDS: Upper tract urothelial carcinoma; epidemiology; ureteric cancer; renal pelvic cancer; nephroureterectomy
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 19–25. https://doi.org/10.2340/sju.v59.16281.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 5 July 2023; Accepted: 20 October 2023; Published: 16 January 2024
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v59.16281
CONTACT Johannes Bobjer johannes.bobjer@med.lu.se Department of Urology, Skåne University Hospital, Jan Waldenströms gata 5, SE-205 02 Malmö, Sweden
Competing interests and funding: The authors have no relevant financial or non-financial interests to disclose.
This work was supported by the Swedish Cancer Society (CAN 2020/0709), Swedish Research Council (2021-00859), Lund Medical Faculty (ALF), Skåne University Hospital Research Funds, the Gyllenstierna Krapperup’s Foundation, The Cancer Research Fund at Malmö General Hospital, Skåne County Council’s Research and Development Foundation (REGSKANE-622351), Gösta Jönsson Research Foundation, the Foundation of Urological Research (Ove and Carin Carlsson bladder cancer donation) and Hillevi Fries Research Foundation. The funding sources had no role in the study design, data analyses, interpretation of the results or writing of the manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) challenges the urologist being a rare disease but also considering a variety of diverse options for both diagnostic planning and treatment. Compared to urothelial carcinoma in the bladder, tumors are more frequently invasive [1] and due to the anatomical nature of the disease, treatment decisions more often have to rely on radiology and cytology rather than pathologic assessment of biopsies. The standard surgical treatment includes radical nephroureterectomy (RNU) for the majority of the patients, with or without concomitant retroperitoneal lymph node dissection (LND) [2]. Organ-sparing strategies with segmental ureteric resection in case of distal ureteral tumor localisation and endoscopic ablative treatment for patients with non-invasive low-risk tumors without signs of high-grade disease remain oncologically feasible alternatives. There is no evidence supporting the routine use of neoadjuvant platinum-based chemotherapy, yet in selected patients with locally advanced and inoperable tumors or in the presence of regional lymph node metastases induction chemotherapy can be considered [3, 4]. After radical surgery, patients possessing high-risk criteria in the tumor specimen are today offered such systemic treatment after radical surgery in the adjuvant setting based on data from the POUT-trial [5]. Adjuvant systemic immunotherapy using the recently introduced check-point inhibitor nivolumab is another postoperative treatment option to consider for high-risk PD-L1-positive patients [6]. Preoperative renal function will also likely affect choice of treatment as 60% of patients become cisplatin-unfit after RNU [7]. Further complicating the diagnostic planning and treatment is the fact that intravesical recurrences after extirpative surgery for UTUC are frequent and increase by applying preoperative invasive diagnostic modalities such as ureteroscopy (URS) with or without biopsy [6]. To improve management and outcomes for patients with UTUC, creation of multidisciplinary tumor boards (MDTB) within existing bladder cancer MDTBs has been suggested [8], in line with the Swedish guideline-recommendation to refer all patients with UTUC to a MDTB prior to invasive diagnostic procedures [9].
Since 2015, patients diagnosed with UTUC are registered in the Swedish National Registry of Urinary Bladder Cancer (SNRUBC). With high coverage, the registry includes information on diagnostic pathways and modalities as well as patient and tumor characteristics and data on treatment [10]. By analysing this nationwide population-based registry, we aimed to explore the current clinical practice regarding UTUC in Sweden in order to reveal trends of diagnostics and treatment to identify where improvements can be made.
Patients and methods
Study population
We identified 2,362 UTUC-patients in the SNRUBC registered from January 2015 when the registration of UTUC started until 2021. After exclusions, 2,124 patients with a primary registration of UTUC remained for evaluation (Figure 1). In case of bilateral synchronous tumors, these patients (n = 43) were excluded from further analyses, as were 95 patients with incidentally detected ureteric cancer in conjunction with radical cystectomy for bladder cancer and 23 patients with missing information on treatment or treatment intent. In case of concomitant registration of ipsilateral tumors in both tumor locations (renal pelvis and ureter, n = 63), patients were referred to as renal pelvic tumors. Data comprise individual patient data registered at diagnosis and treatment including a separate form for oncological treatment. All data relies on adherence to national guidelines for submission of the registration forms by the local hospital.
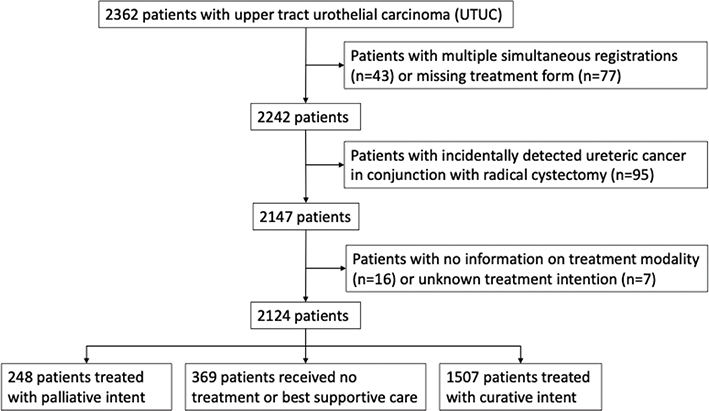
Figure 1. CONSORT-diagram of the study population.
Outcome measures
Data were retrieved regarding age at diagnosis, sex, health-care region, usage of MDTB, clinical TNM-stage and grade (WHO 1999), tumor location, dates of referral/first visit at a specialist in urology/diagnosis (either clinically by radiology or by biopsy, whatever occurred first) and treatment. Additionally, diagnostic modalities applied were obtained together with data regarding treatment such as surgical approach (open, laparoscopic or robot), type of extirpative surgery (RNU or segmental ureteric resection) or endoscopic treatment and/or instillation therapy as well as use of systemic chemotherapy. Registration of treatment include an obligation to specify if treatment has been given with an intent to cure or with a non-curative or palliative intent. In Sweden, standardized care pathways (SCP) for bladder cancer (including UTUC) were fully implemented in 2016 [11] and, being a quality indicator, information whether SCP was applied or not was also retrieved from the SNRUBC. Other quality indicators regarding extirpative UTUC surgery [12], such as distal ureter management with excision of the ipsilateral distal ureter with a bladder cuff and delivery of systemic chemotherapy, were also retrieved from the SNRUBC, although information about extent of LND and use of postoperative single-dose instillation after RNU was lacking.
Statistical analyses
Baseline characteristics are presented as medians with interquartile ranges (IQR) and proportions (%) stratified by tumor location in the renal pelvis and ureter, respectively. For relevant variables descriptive data are presented separately for patients who received treatment with curative intention and patients who either received no treatment or treatment without curative intention (palliative care). Incidence rates per 100,000 population standardised by age and sex were computed per year for the total study population and separately for UTUC in the renal pelvis and ureter. Incidence trend stratified by years was tested with a simple T-test. Lead times were computed based on days from date of referral to a) first visit at specialist, b) date of diagnosis and c) treatment date. A Wilcoxon rank sum test was applied to compare diagnostic and treatment delays in 2015 (prior to initiation of SCP) versus 2016–2021. Logistic regression analysis was used to assess changes in MDTB usage by study years. P-values were computed using a Wald test on the adjusted logistic model.
Patients were stratified in groups based on increasingly invasive diagnostic modalities (IDM) as follows: [13] In addition to cystoscopy and a computed tomography (CT) urography or a magnetic resonance tomography (MRT), either A) voided urine cytology (reference group with no instrumentation of the upper tract), B) retrograde/antegrade pyelography and/or selective urine cytology, C) ureteropyeloscopy with or without barbotage for cytology, or D) ureteropyeloscopy with tumor biopsy. If patients matched several criteria, they were categorized according to the most invasive modality (e.g. one patient with both A and B was categorized as B in the calculations). To explore trends in diagnostics and treatment, difference in proportions of various study variables between groups by study years were computed using Chi-2 tests.
For all statistical analyses, the R statistical package version 4.2.3 was used [14].
Ethical review
The study was approved by the Research Ethics Board of Uppsala University, Sweden (EPN 2023-04690-02).
Results
Baseline patient characteristics are presented in Table 1. Median age was 74 (IQR 70–77) and 75 (IQR 71–78) years for the 1,213 and 911 patients with UTUC located in the renal pelvis and ureter, respectively.
| All patients | Curative treatment intention | Palliative treatment or best supportive care | ||||||||
| Renal pelvis N = 1,213 | Ureter N = 911 | Renal pelvis N = 872 | Ureter N = 635 | Renal pelvis N = 341 | Ureter N = 276 | |||||
| Age at diagnosis | Median | 74 | 75 | 73 | 74 | 75 | 75 | |||
| IQR | 70–77 | 71–78 | 70–77 | 71–78 | 71–78 | 71–79 | ||||
| No diagnosed per year | 2015 | 171 (14%) | 124 (14%) | 129 (15%) | 83 (13%) | 42 (12%) | 41 (15%) | |||
| 2016 | 195 (16%) | 116 (13%) | 139 (16%) | 79 (12%) | 57 (17%) | 37 (13%) | ||||
| 2017 | 184 (15%) | 138 (15%) | 146 (17%) | 105 (17%) | 38 (11%) | 33 (12%) | ||||
| 2018 | 163 (13%) | 151 (17%) | 117 (13%) | 109 (17%) | 46 (13%) | 44 (16%) | ||||
| 2019 | 169 (14%) | 145 (16%) | 112 (13%) | 96 (15%) | 58 (17%) | 49 (18%) | ||||
| 2020 | 176 (14%) | 133 (15%) | 121 (14%) | 86 (14%) | 55 (16%) | 47 (17%) | ||||
| 2021a | 155 (13%) | 104 (11%) | 108 (12%) | 77 (12%) | 47 (14%) | 27 (9.7%) | ||||
| Sex | Male | 725 (60%) | 585 (64%) | 528 (61%) | 409 (64%) | 197 (57%) | 176 (63%) | |||
| Female | 490 (40%) | 328 (36%) | 344 (39%) | 226 (36%) | 146 (43%) | 102 (37%) | ||||
| Health-care region | Stockholm/Gotland | 255 (21%) | 224 (25%) | 189 (22%) | 167 (26%) | 66 (19%) | 57 (21%) | |||
| Uppsala/ Örebro | 260 (21%) | 140 (15%) | 169 (19%) | 81 (13%) | 91 (27%) | 59 (21%) | ||||
| South-eastern | 124 (10%) | 99 (11%) | 96 (11%) | 74 (12%) | 28 (8.2%) | 25 (9.0%) | ||||
| Southern | 239 (20%) | 211 (23%) | 193 (22%) | 157 (25%) | 46 (13%) | 56 (20%) | ||||
| Western | 235 (19%) | 157 (17%) | 150 (17%) | 96 (15%) | 85 (25%) | 61 (22%) | ||||
| Northern | 100 (8.2%) | 80 (8.8%) | 75 (8.6%) | 60 (9.4%) | 25 (7.3%) | 20 (7.2%) | ||||
| cT-stage | Cis | 14 (1.2%) | 53 (5.8%) | 12 (1.4%) | 36 (5.7%) | 2 (0.6%) | 17 (6.2%) | |||
| Ta | 444 (37%) | 463 (51%) | 361 (41%) | 326 (51%) | 83 (24%) | 137 (50%) | ||||
| T1 | 152 (13%) | 141 (15%) | 118 (14%) | 97 (15%) | 34 (10%) | 44 (16%) | ||||
| T2 | 94 (7.7%) | 86 (9.4%) | 77 (8.8%) | 66 (10%) | 17 (5.0%) | 20 (7.2%) | ||||
| T3 | 314 (26%) | 104 (11%) | 213 (24%) | 77 (12%) | 101 (30%) | 27 (9.8%) | ||||
| T4 | 97 (8.0%) | 12 (1.3%) | 44 (5.0%) | 7 (1.1%) | 53 (16%) | 5 (1.8%) | ||||
| Tx | 98 (8.1%) | 52 (5.7%) | 47 (5.4%) | 26 (4.1%) | 51 (15%) | 26 (9.4%) | ||||
| cN-stage | N0 | 834 (69%) | 678 (74%) | 674 (77%) | 487 (77%) | 160 (47%) | 191 (69%) | |||
| N1 | 71 (5.9%) | 19 (2.1%) | 35 (4.0%) | 9 (1.4%) | 36 (11%) | 10 (3.6%) | ||||
| N2 | 119 (9.8%) | 32 (3.5%) | 32 (3.7%) | 11 (1.7%) | 87 (26%) | 21 (7.6%) | ||||
| Nx | 189 (16%) | 182 (20%) | 131 (15%) | 128 (20%) | 58 (17%) | 54 (20%) | ||||
| cM-stage | M0/Mxb | 1,072 (88%) | 871 (96%) | 843 (97%) | 626 (99%) | 229 (67%) | 245 (89%) | |||
| M1 | 141 (12%) | 40 (4.4%) | 29 (3.3%) | 9 (1.4%) | 112 (33%) | 31 (11%) | ||||
| Clinical grade | LMP | 9 (0.7%) | 16 (1.8%) | 7 (0.8%) | 9 (1.4%) | 2 (0.6%) | 7 (2.5%) | |||
| G1 | 150 (12%) | 195 (21%) | 117 (13%) | 127 (20%) | 33 (9.7%) | 68 (25%) | ||||
| G2 | 370 (31%) | 291 (32%) | 292 (34%) | 213 (34%) | 78 (23%) | 78 (28%) | ||||
| G3 | 561 (46%) | 365 (40%) | 390 (45%) | 258 (41%) | 171 (50%) | 107 (39%) | ||||
| Gx | 123 (10%) | 44 (4.8%) | 66 (7.6%) | 28 (4.4%) | 57 (17%) | 16 (5.8%) | ||||
| aNot including a full year. bNo thorax radiology performed. |
||||||||||
Incidence
The total number of UTUC diagnoses per year is available in Table 1. UTUC incidence, standardised by age and sex, remained unchanged in Sweden during the study period (Table 2).
| Total | Renal pelvis | Ureter | ||||||||
| 2015 | 2.99 | 1.74 | 1.26 | |||||||
| 2016 | 3.10 | 1.94 | 1.16 | |||||||
| 2017 | 3.14 | 1.79 | 1.35 | |||||||
| 2018 | 2.99 | 1.56 | 1.43 | |||||||
| 2019 | 2.90 | 1.55 | 1.36 | |||||||
| 2020 | 2.83 | 1.64 | 1.19 | |||||||
| p1 | 0.108 | 0.142 | 0.736 | |||||||
| 1Trend tested with a simple T-test. 2021 did not include a full year. | ||||||||||
Lead times and diagnostics
Lead times and diagnostic modalities are presented in Table 3 for the total study cohort. A SCP was applied in 1,030/2,124 (48%) of the patients diagnosed with UTUC, and 401 (19%) were referred to another hospital for treatment. The median number of days from referral to diagnosis was 14 (IQR 5–26) and 16 (IQR 5–31) days for UTUC of the renal pelvis and ureter, respectively. The corresponding median number of days from referral to surgical treatment was 76 (IQR 57–99) and 90 (IQR 72–117) days, respectively. Comparing lead time from referral to treatment for patients diagnosed in 2015 before initiating SCP with those diagnosed 2016–2021, showed no difference (82 [IQR 56–129] vs. 79 [IQR 51–121] days, p = 0.35). The corresponding lead time before and after the initiation of SCP for patients in the subgroups with renal pelvic cancer and ureteric cancer were 81 (IQR 56–131) versus 75 (IQR 48–109) days (p = 0.054) and 89 (IQR 55–125) versus 89 (IQR 61–134) days (p = 0.33), respectively. The proportion of patients discussed in a MDTB was 1,419/2,124 (67%) for the total study period and 1,001/1,507 (66%) in the subset of patients where curative treatment intent had been specified. A continuously increasing proportion of patients was discussed at a MDTB over the included study years (Figure 2a).
| Renal pelvis N = 1,213 | Ureter N = 911 | |||||||||
| Standardized care pathwaya | Yes | 638 (62%) | 392 (50%) | |||||||
| Missing | 176 | 127 | ||||||||
| Referral to other hospital for treatment | Yes | 235 (19%) | 166 (18%) | |||||||
| Days from referral to first visit at specialist | Median | 12 | 9 | |||||||
| IQR | 7–16 | 3–15 | ||||||||
| Missing | 16 | 18 | ||||||||
| Days from referral to diagnosis | Median | 14 | 16 | |||||||
| IQR | 5–26 | 5–31 | ||||||||
| Missing | 12 | 17 | ||||||||
| Days from referral to treatment (RNUb or segmental resection or endoluminal resection) | Median | 76 | 90 | |||||||
| IQR | 57–99 | 72–117 | ||||||||
| Missing | 390 | 298 | ||||||||
| MDTBc | Yes | 792 (66%) | 627 (70%) | |||||||
| Missing | 7 | 13 | ||||||||
| IDM groupsd | A | 531 (44%) | 401 (44%) | |||||||
| B | 68 (5.6%) | 54 (5.9%) | ||||||||
| C | 99 (8.2%) | 49 (5.4%) | ||||||||
| D | 515 (42%) | 407 (45%) | ||||||||
| aOnly patients diagnosed 2016 and after; bRNU = radical nephroureterectomy; cMDTB = multidisciplinary tumor board; dIDM = invasive diagnostic modalities: A) voided urine cytology or radiology only, B) retrograde/antegrade pyelography and/or selective urine cytology, C) ureteropyeloscopy with or without barbotage for cytology or D) ureteropyeloscopy with tumor biopsy | ||||||||||
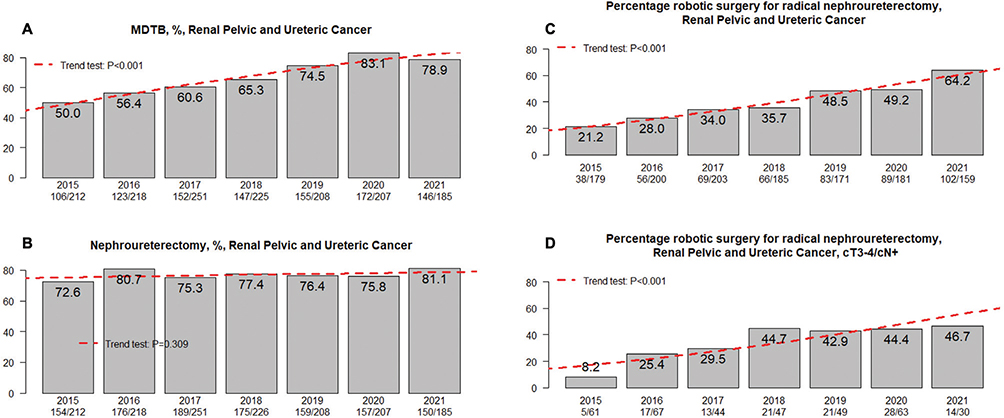
Figure 2. (A) Proportion of multidisciplinary tumor board (MDTB) usage per year. (B) Proportion of radical nephroureterectomy (RNU) per year. (C) Proportion of robot assisted RNU per study year for the total population and (D) for clinically non-organ confined (cT3-4) and/or node positive (cN+) disease.
The proportion of patients stratified in groups based on degree of preoperative IDM and tumor location is presented in Table 3. In the total study population 531/1213 (44%) and 401/911 (44%) of patients with renal pelvic or ureteric cancer were diagnosed based on cystoscopy, voided urine cytology and imaging only (IDM group A). No significant change in IDM usage was noted during the included study period and when assessing patients with curative intent only a registration of MDTB did not influence the proportion of IDM usage (data not shown).
Treatment
Curatively intended treatment was registered for 872/1,213 (72%) and 635/911 (70%) of patients with renal pelvic and ureteric tumor location, respectively. These proportions did not change between study years (data not shown). For these patients, organ-preserving surgery was registered for renal pelvis tumor location in 59/872 (6.8%) of whom 50 (5.7%) were treated with endoluminal resection/ablation and nine (1.0%) with local resection. Whether these nine individuals were treated with percutaneous resection or open resection with or without renal auto-transplantation is not known. For ureteric tumor location organ-preserving treatment was registered in 212/635 (33%) patients, of which 72 (11%) were treated with endoluminal resection/coagulation and 140 (22%) with segmental ureteric resection. Additionally, BCG instillation for UTUC was registered for eight and 17 patients with renal pelvic and ureteric tumor location, respectively. The proportion of patients subjected to RNU by study year did not reveal any significant changes (Figure 2b). RNU stratified by tumor location (renal pelvis and ureter) was performed with laparoscopy in 141/816 (17%) and 53/464 (11%), open surgery in 359/816 (44%) and 222/464 (48%) and robot-assisted technique in 316/816 (39%) and 189/464 (41%) of patients. When these surgical approaches for RNU were stratified by year of treatment, a shift towards more robotic surgery over time was evident (Figure 2c). During study years, an increasing proportion of robot assisted RNU was observed for patients with clinically locally advanced cT3-4 and/or cN+ (n = 528) disease (Figure 2d). Treatment details in the subset of patients with no registration of curative intention are available in Table S1.
Treatment quality indicators
Separate analysis in the subset of patients that were subjected to RNU (n = 1,280) showed registration of an ipsilateral bladder cuff excision during the extirpation of the distal ureter in 522/816 (64%) and 308/464 (67%) of patients with renal pelvic and ureteric tumor location, respectively. Systemic oncological treatment was registered for 213 patients in the total population (induction n = 19, neoadjuvant n = 22, adjuvant n = 81, palliative n = 79, not specified/other n = 12). For patients subjected to RNU or segmental ureteric resection with pT2-4 and/or pN+ disease in the resected specimen, adjuvant systemic chemotherapy was registered in 62/592 (11%) patients. Stratifying such adjuvant treatment before and after this was included in national guideline recommendations in 2019, showed an increase from 20/342 (5.8%) in 2015–2018 to 42/250 (17%) between 2019 and 2022.
Clinical versus pathological TNM-stage and grade
Registrations of pathological tumor TNM-stage and grade is available in Table S2. For the 427 patients where a preoperative biopsy was undertaken (IDM group D) before RNU or segmental ureteric resection, pT-stage was higher compared to the clinical tumor stage based on radiology and findings in endoscopically obtained biopsies in 128 (27%) individuals, whereas downstaging occurred in 11/467 (2.4%) individuals (Table S3). Similarly, upgrading in relation to endoscopically obtained biopsies was registered in 72/467 (15%) individuals compared to downgrading in 28/467 (5.9%) (Table S4).
Discussion
The Swedish SNRUBC provides key information on UTUC since the start of registration in 2015 and improves the understanding of how this rare disease entity is diagnosed and treated, together with other available UTUC registries [15]. This initial report on UTUC in SNRUBC identifies some key areas where improvements are needed. The surprisingly high proportion of patients not subjected to curatively intended treatment together with median lead-times from referral to treatment exceeding those recommended are two such findings. Furthermore, the lack of a complete distal ureteric excision with a bladder cuff in one out of three patients subjected to RNU stands out together with a seemingly limited use of systemic chemotherapy in conjunction with surgery, even though the proportion of patients receiving adjuvant chemotherapy increased after 2019.
The current study holds limitations that need to be considered. Register-based data may suffer from systematic reporting bias that for some variables could be more pronounced due to for example non-intuitive reporting forms. Additionally, with the registration form applied during the study years, the proportion of individuals subjected to LND (and the extent of LND) is unknown. Moreover, lack of details for some variables limits the possibility to draw firm conclusions of the retrieved data, as in the assessment of the proportion of patients discussed at MDTB where we lack information about when this occurred during the diagnostic process. As a result, it is difficult to determine the impact of MDTB on the initiation of invasive diagnostic procedures or kidney sparing strategies. Still, nation-wide registers like the present allow for assessment of real-world data in larger study groups, which is favourable in a low incidence disease such as UTUC. Also, the current study is strengthened by the previously reported high coverage in the SNRUBC [10].
Exploring incidence trends in Sweden during the study years 2015–2020 revealed stable levels of UTUC diagnoses. Compared to other contemporary population-based reports, data from the Netherlands showed an increased incidence of UTUC from 1993 to 2017 [16], whereas data from NHS England 2013–2019 showed stable UTUC incidence [17], and US data from 2004 to 2016 showed a slight decrease, yet with an increasing proportion of primary metastatic disease during the study years [18]. Another key observation is the relatively high proportion of patients (617/2,124 [29%]) diagnosed with UTUC in the current series that for some reason could not receive treatment with curative intent, compared to 15% in a recent tertial referral center study [19], highlighting the importance of population-based registries for exploring real-world trends.
The nationwide introduction of SCP tended to shorten the total delay from referral to treatment for patients with renal pelvic cancer. Still, a median total delay from referral to surgical treatment of 76 and 90 days for renal pelvis and ureteric cancer means that the majority of patients were operated with a delay above the proposed limits of 1 month for ureteric cancer and 2 months in patients with hydronephrosis, after which worse survival outcome has been reported [20]. The identified increased use of MDTB was observed during the study period is in line with guidelines [9]. Other quality indicators such as excision of an ipsilateral bladder cuff in conjunction with RNU displayed a lower extent of guideline adherence with a bladder cuff excised in less than two out of three patients despite a high risk of ureteral stump recurrence in up to 30% of these patients [21]. However, there are possibly other ways of evaluating and reporting distal ureter management rather than reports from individual urologists that would better reflect this variable such as registration of a ureteric orifice remnant at cystoscopic follow-up.
The risk of upstaging in the radically resected UTUC-specimen in the current nation-wide study was less frequent compared to a recent multicenter study reporting upstaging in 60% of all patients [22]. Similarly, upgrading in the pathologic specimen occurred only in 15% of the patients in the current series compared to 42% in the multicenter study by Mori et al. [22]. Possibly, the differences could be explained by differences regarding clinical workup and diagnostic strategies. In the current population-based data, less than half of all patients were subjected to IDM with ureteroscopic biopsies, compared to all patients in the multicenter trial, implying selection mechanisms. Nonetheless, the notion that preoperative staging in UTUC is associated with a high level of uncertainty remains, stressing the importance of close co-operation with dedicated uro-pathologists and uro-radiologists in a MDTB setting.
Surgical approach for RNU during the study years adheres to a global trend of more robotic technique in favor of conventional laparoscopy and open surgery [23]. Current guidelines recommend an open approach in non-organ-confined or clinically node positive disease [3], based on weak evidence [24]. Still, one out of three patients subjected to RNU for cT3-4 and/or cN+ disease were operated with robotic technique in the current data and almost half of these patients during latter study years (Figure 2d), suggesting that advantages in perioperative outcomes and an increased familiarity with a technique has led to expansion of indications of its usage. Further studies are needed to decide whether a robotic approach can yield non-inferior oncological results also in high-risk patients. This is also an issue in terms of distal ureter management where the minimally invasive extravesical approach has been linked to increased rates of intravesical recurrences [25].
Recommendations for systemic treatment changed during the included study years, as adjuvant platinum-based chemotherapy became standard treatment in case of >pT1 and/or pN+ based on the POUT-trial published in 2020 [5]. In Sweden, clinical implementation started in 2019, which mirrors the marked increase in usage from that year onward in our data (5.8% 2015–2019 vs. 17% 2019–2022). Thus, compared to contemporary data from NHS England 2013–2019 where 20% of patients with muscle invasive UTUC received systemic chemotherapy in conjunction with surgery [17], our present data reveal a more conservative attitude. Also noteworthy, the current study reflects treatment before adjuvant nivolumab was introduced as an option for high-risk PD-L1 positive patients [6]. Albeit growing, the use of pre- and postoperative systemic therapy in our national data pinpoints another area of UTUC treatment that can and should be improved.
Conclusion
The present data give insights into contemporary trends in UTUC diagnostics and treatment in Sweden. Albeit with limitations in terms of both possible misclassification and lack of important variables, we identified a surprisingly high proportion of patients that did not receive treatment with curative intent (29%), and treatment lead times beyond current recommendations. Assessment of oncological outcomes and survival will be possible through additional follow-up studies, allowing for further investigations of the concerns addressed in the current work.
ORCID
Fredrik Liedberg  https://orcid.org/0000-0001-8193-0370
https://orcid.org/0000-0001-8193-0370
Truls Gårdmark  https://orcid.org/0000-0003-4610-0771
https://orcid.org/0000-0003-4610-0771
Staffan Jahnson  https://orcid.org/0000-0001-8012-2742
https://orcid.org/0000-0001-8012-2742
Viveka Ströck  https://orcid.org/0000-0003-2050-6466
https://orcid.org/0000-0003-2050-6466
Karin Söderkvist  https://orcid.org/0000-0002-3683-3763
https://orcid.org/0000-0002-3683-3763
Johannes Bobjer  https://orcid.org/0000-0001-8495-3498
https://orcid.org/0000-0001-8495-3498
References
- [1] Margulis V, Shariat S, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–33. https://doi.org/10.1002/cncr.24135
- [2] Roupret M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2020 Update. Eur Urol. 2021;79(1):62–79. https://doi.org/10.1016/j.eururo.2020.05.042
- [3] Roupret M, Seisen T, Birtle AJ, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2023 Update. Eur Urol. 2023 Jul;84(1):49-64. doi: https://doi.org/10.1016/j.eururo.2023.03.013
- [4] Shigeta K, Matsumoto K, Ogihara K, et al. Does neoadjuvant chemotherapy have therapeutic benefit for node-positive upper tract urothelial carcinoma? Results of a multi-center cohort study. Urol Oncol. 2022;40(3):105.e19–105.e26. https://doi.org/10.1016/j.urolonc.2021.07.029
- [5] Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–77. https://doi.org/10.1016/S0140-6736(20)30415-3
- [6] Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–14. https://doi.org/10.1056/NEJMoa2034442
- [7] Lane BR, Smith AK, Larson BT, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967–73. https://doi.org/10.1002/cncr.25043
- [8] Tay LJ, Chatterton K, Colemeadow J, et al. Improving management of upper tract urothelial carcinoma. BJU Int. 2020;126(1):5–6. https://doi.org/10.1111/bju.15068
- [9] Liedberg F, Kjellstrom S, Lind AK, et al. Swedish national guidelines on urothelial carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand J Urol. 2022 Apr;56(2):137-146. doi: https://doi.org/10.1080/21681805.2022.2041086.
- [10] Haggstrom C, Liedberg F, Hagberg O, et al. Cohort profile: the Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the Bladder Cancer Data Base Sweden (BladderBaSe). BMJ Open. 2017;7(9):e016606. https://doi.org/10.1136/bmjopen-2017-016606
- [11] Abuhasanein S, Jahnson S, Aljabery F, et al. Standardized care pathways for patients with suspected urinary bladder cancer: the Swedish experience. Scand J Urol. 2022;56(3):227–32. https://doi.org/10.1080/21681805.2022.2058605
- [12] Konig F, Shariat SF, Karakiewicz PI, et al. Quality indicators for the management of high-risk upper tract urothelial carcinoma requiring radical nephroureterectomy. Curr Opin Urol. 2021;31(4):291–6. https://doi.org/10.1097/MOU.0000000000000895
- [13] Liedberg F, Hagberg O, Haggstrom C, et al. Preoperative upper tract invasive diagnostic modalities are associated with intravesical recurrence following surgery for upper tract urothelial carcinoma: a population-based study. PLoS One. 2023;18(2):e0281304. https://doi.org/10.1371/journal.pone.0281304
- [14] R Core team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/
- [15] Kealey J, Snider R, Hayne D, et al. The utility of clinical registries for guiding clinical practice in upper tract urothelial cancer: a narrative review. Transl Androl Urol. 2023;12(3):497–507. https://doi.org/10.21037/tau-22-641
- [16] van Doeveren T, van der Mark M, van Leeuwen PJ, et al. Rising incidence rates and unaltered survival rates for primary upper urinary tract urothelial carcinoma: a Dutch population-based study from 1993 to 2017. BJU Int. 2021;128(3):343–51. https://doi.org/10.1111/bju.15389
- [17] Catto JWF, Mandrik O, Quayle LA, et al. Diagnosis, treatment and survival from bladder, upper urinary tract and urethral cancers: real world findings from NHS England between 2013 and 2019. BJU Int. 2023 Jun;131(6):734-744. doi: 10.1111/bju.15970. Epub 2023 Feb 7. https://doi.org/10.1111/bju.15970
- [18] Colla Ruvolo C, Nocera L, Stolzenbach LF, et al. Incidence and survival rates of contemporary patients with invasive upper tract urothelial carcinoma. Eur Urol Oncol. 2021;4(5):792–801. https://doi.org/10.1016/j.euo.2020.11.005
- [19] Kealey J, Ip C, Davis ID and Sengupta S. The impact of multidisciplinary cancer meetings in guiding treatment intent in patients with upper tract urothelial carcinoma. Asia Pac J Clin Oncol. 2023 Mar 31. doi: 10.1111/ajco.13952. https://doi.org/10.1111/ajco.13952
- [20] Nowak L, Krajewski W, Laszkiewicz J, et al. The impact of surgical waiting time on oncological outcomes in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy: a systematic review. J Clin Med. 2022 2022 Jul 11;11(14):4007. doi: 10.3390/jcm11144007. https://doi.org/10.3390/jcm11144007
- [21] Strong DW, Pearse HD, Tank ES Jr, et al. The ureteral stump after nephroureterectomy. J Urol. 1976;115(6):654–5. https://doi.org/10.1016/S0022-5347(17)59324-6
- [22] Mori, K Katayama S, Laukhtina E, et al. Discordance between clinical and pathological staging and grading in upper tract urothelial carcinoma. Clin Genitourin Cancer. 2022;20(1):95.e1–95.e6. https://doi.org/10.1016/j.clgc.2021.10.002
- [23] Tinay I, Gelpi-Hammerschmidt F, Leow JJ, et al. Trends in utilisation, perioperative outcomes, and costs of nephroureterectomies in the management of upper tract urothelial carcinoma: a 10-year population-based analysis. BJU Int. 2016;117(6):954–60. https://doi.org/10.1111/bju.13375
- [24] Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological outcomes of laparoscopic nephroureterectomy versus open radical nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology guidelines systematic review. Eur Urol Focus. 2019;5(2):205–23. https://doi.org/10.1016/j.euf.2017.10.003
- [25] Seisen T, Granger B, Colin P, et al. A systematic review and meta-analysis of clinicopathologic factors linked to intravesical recurrence after radical nephroureterectomy to treat upper tract urothelial carcinoma. Eur Urol. 2015;67(6):1122–33. https://doi.org/10.1016/j.eururo.2014.11.035