ORIGINAL RESEARCH ARTICLE
The association between age and long-term quality of life after curative treatment for prostate cancer: a cross-sectional study
Reidun Sletten a,b,c*, Ola Berger Christiansen
a,b,c*, Ola Berger Christiansen a,d, Line Merethe Oldervoll
a,d, Line Merethe Oldervoll c,e, Lennart Åstrøm
c,e, Lennart Åstrøm f, Håvard Kjesbu Skjellegrind
f, Håvard Kjesbu Skjellegrind g,h, Jūratė Šaltytė Benth
g,h, Jūratė Šaltytė Benth a,i,j, Øyvind Kirkevold
a,i,j, Øyvind Kirkevold a,k,l, Sverre Bergh
a,k,l, Sverre Bergh a,l, Bjørn Henning Grønberg
a,l, Bjørn Henning Grønberg m,n, Siri Rostoft
m,n, Siri Rostoft o,p, Asta Bye
o,p, Asta Bye q, Paul Jarle Mork
q, Paul Jarle Mork c and Marit Slaaen
c and Marit Slaaen a,o
a,o
aThe Research Center for Age-Related Functional Decline and Disease, Innlandet Hospital Trust, Ottestad, Norway; bDepartment of Oncology and Palliative Care, Innlandet Hospital Trust, Gjøvik/Lillehammer, Norway; cDepartment of Public Health and Nursing, Norwegian University of Science and Technology (NTNU), Trondheim, Norway; dDepartment of Urology, Innlandet Hospital Trust, Hamar, Norway; eCentre for Crisis Psychology, Faculty of Psychology University of Bergen, Bergen, Norway; fSection of Clinical and Experimental Oncology, Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden; gHUNT Research Centre, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, NTNU, Levanger, Norway; hLevanger Hospital, Nord-Trøndelag Hospital Trust, Levanger, Norway; iInstitute of Clinical Medicine, Campus Ahus, University of Oslo, Oslo, Norway; jHealth Services Research Unit, Akershus University Hospital, Lørenskog, Norway; kFaculty of Health, Care and Nursing, NTNU Gjøvik, Gjøvik, Norway; lThe Norwegian National Centre for Ageing and Health, Tønsberg, Norway; mDepartment of Clinical and Molecular Medicine, NTNU, Trondheim, Norway; nDepartment of Oncology, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; oInstitute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; pDepartment of Geriatric Medicine, Oslo University Hospital, Oslo, Norway; qOslo Metropolitan University, Oslo, Norway
ABSTRACT
Objective: We aimed to investigate the associations between age at radical prostate cancer treatment and long-term global quality of life (QoL), physical function (PF), and treatment-related side effects.
Material and Methods: This single-center, cross-sectional study included men treated for localized prostate cancer with robotic-assisted radical prostatectomy (RARP) or external beam radiotherapy (EBRT) in 2014–2018. Global QoL and PF were assessed by the European Organisation of Research and Treatment in Cancer Quality of life Questionnaire-C30 (QLQ-C30), side effects by the Expanded Prostate Cancer Index Composite (EPIC-26). Adjusted linear regression models were estimated to assess associations between age (continuous variable) at treatment and outcomes. QLQ-C30 scores were compared to normative data after dividing the cohort in two groups, <70 years and ≥70 years at treatment.
Results: Of 654 men included, 516 (79%) had undergone RARP, and 138 (21%) had undergone EBRT combined with androgen deprivation therapy for 93%. Mean time since treatment was 57 months. Median age at treatment was 68 (min–max 44–84) years. We found no statistically significant independent association between age at treatment and global QoL, PF or side effects, except for sexual function (regression coefficient [RC] −0.77; p < 0.001) and hormonal/vitality (RC 0.30; p = 0.006) function. Mean QLQ-C30 scores were slightly poorer than age-adjusted normative scores, for men <70 years (n = 411) as well as for men ≥70 years (n = 243) at treatment, but the differences were not beyond clinical significance.
Conclusions: In this cohort of prostate cancer survivors, age at treatment had little impact on long-term QoL and function. Due to the cross-sectional design, short term impact or variation over time cannot be ruled out.
KEYWORDS: Prostate cancer; radical treatment; quality of life; older patients
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 31–38. https://doi.org/10.2340/sju.v59.18616.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 1 September 2023; Accepted: 23 January 2024; Published: 20 February 2024
CONTACT Reidun Sletten reidun.sletten@ntnu.no Department of Oncology and Palliative Care, Innlandet Hospital Trust, Kyrre Grepps gate 11, NO-2819 Gjøvik, Norway
Competing interests and funding: The authors report no conflicts of interest.
This work was supported by Innlandet Hospital Trust, under Grant 150410.
Introduction
Organ-confined prostate cancer is potentially curable, and men with an expected survival of 10 years or more are candidates for treatment with curative intent [1]. Two main options are available, namely surgery or radiotherapy, the latter most often combined with androgen deprivation therapy (ADT). Both surgery and radiotherapy commonly have a negative impact on overall quality of life (QoL) as well as physical and emotional functioning [2–4]. Surgery often causes side effects like urinary incontinence and erectile dysfunction, whereas radiotherapy more often causes irritative/obstructive urinary complaints and bowel discomfort. General side effects like fatigue and psychological distress are seen after both modalities [2–4].
The median age at diagnosis of prostate cancer in Norway is 70 years [5]. Older men are diagnosed with more advanced disease and have higher disease-specific mortality. Despite documented benefits in terms of reduced mortality and morbidity from advanced disease [6, 7], older men are still less likely to receive curative treatment [8, 9]. Age seems to be the most important factor in deciding not to offer curative treatment [10]. Concerns regarding side effects and negative impact on QoL might be the cause.
Older patients are underrepresented in clinical trials [11], and studies addressing older prostate cancer survivors are often limited by lack of comparison to their younger counterparts. The majority of studies investigating the impact of radical prostate cancer treatment on patient reported outcomes (PROs) have focused on treatment-related side effect, that is, functional outcomes related to the prostatic area and adjacent organs [12]. Few have looked at other QoL dimensions. General well-being and independence are crucially important to older adults [13, 14]; but despite this, the knowledge on how older prostate cancer survivors experience their overall QoL and physical function (PF) is scarce. Moreover, the overall results regarding the impact of age at treatment are not consistent. There are several reports that older men may fare worse, in particular with respect to some local side effects [15–17], but others have found that functioning and QoL are mainly preserved [18–21]. Thus, for patient information and shared decision making, there is a need for more knowledge on how this older group tolerates curative prostate cancer treatment, in particular as their number is likely to increase due to an ageing population.
The main objective of this paper was to investigate whether self-reported global quality of life (global QoL) and PF differ according to age at the time of curative treatment for prostate cancer. In addition, we investigated the association between age at treatment and late treatment-related side effects.
Material and methods
Setting/context
This is a single-center study in a public hospital with a catchment area of about 370,000 inhabitants. Approximately 280 prostate cancer patients in this area receive curative treatment every year.
Study design and patients
This is a cross-sectional study of Norwegian-speaking men receiving curative treatment for prostate cancer between January 2014 and December 2018. Eligible men identified by the hospital’s electronic medical record were invited to participate and consented by mail in May 2021.
The men received either external beam radiotherapy (EBRT) or robotic-assisted radical prostatectomy (RARP). Treatment for individual patients was selected based on multidisciplinary team discussions and according to the guidelines prepared by the European Association of Urology [1]. As there is no definite consensus on the choice between either RARP or EBRT for older patients, allocation to treatment modality was by surgeons’ and/or patients’ choice, based on judgement of factors as operability and comorbidities, and on patients’ preferences. EBRT was given as 74–78 Gy in 37–38 fractions or 60 Gy in 20 fractions. If not contra-indicated or refused by the patient, EBRT was combined with ADT either neoadjuvant as a luteinizing hormone-releasing hormone (LHRH) agonist, or combined neoadjuvant and adjuvant treatment, the latter given as a LHRH agonist or an antiandrogen. RARP was performed using the da Vinci Surgical System®. An intended nerve-sparing procedure was performed if extra-prostatic extension was absent. In most cases, patients with an estimated risk of having locally advanced disease with lymph node involvement had extended pelvic lymph node dissection for pathological staging.
Assessment
The men participating in the study completed questionnaires on QoL, sociodemographic and medical history data, including the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 (QLQ-C30) [22] and the 26-item short form version of the Expanded Prostate Cancer Index Composite (EPIC-26) [23, 24]. The QLQ-C30 consists of 30 items covering five functioning scales (physical, role, emotional, cognitive and social), three symptom subscales (fatigue, nausea/vomiting and pain), six single items (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties) and a global QoL scale (global QoL). All items have response categories ranging from 0 (not at all) to 4 (very much) except the two global QoL items, which are scored 0 (very poor) to 7 (excellent). Before analyses, scores for each scale/item were transformed to scales ranging from 0 to 100. Higher scores on the functioning and global QoL scales represent better function, whereas higher scores on the symptom scales represent more symptoms. A difference in mean score of 10 or more is considered clinically significant [25, 26]. We used the latest published normative data for the Norwegian population for comparison [27]. This sample included 1127 men from 19 to 79 years old [mean (SD) 55 (15.5) years]; 238 men were between 70 and 79 years.
The EPIC-26 is a validated questionnaire, widely used to measure local side effects and symptoms after prostate cancer treatment [23, 24]. It contains 26 items covering five domains: urinary incontinence, urinary irritative/obstructive, bowel, sexual and vitality/hormonal. Each item is a four- or five-point Likert scale with explanatory text. The scores on each item are standardized, and multi-item scale scores are transformed linearly to a scale ranging from 0 to 100, where higher scores mean more symptoms. Minimally important difference (MID) in mean scores is reportedly 4–6 points for bowel and vitality/hormonal domains, 5–7 for urinary irritation/obstruction, 6–9 for urinary incontinence, and 10–12 points for sexual domain [28].
Information on comorbidities, prostate cancer characteristics, cancer treatment and whether relapses occurred was obtained from patients’ electronic medical records. Charlson Comorbidity Index (CCI) without age adjustment was used to score comorbidity [29, 30]. Low, intermediate and high-risk prostate cancer [1] was grouped based on tumour stage, histological grade in primary biopsies, and prostatic specific antigen (PSA). Clinical relapse was defined as the occurrence of distant metastases or additional treatment (salvage radiotherapy or surgery, lifelong ADT, chemotherapy or other medical treatment for recurrence).
Ethics
The project was approved by the Regional Committee for Medical Research Ethics South East Norway (REK South East) (ID 183868, on 10th of March 2021) and the local, official data protection officer, and registered in ClinicalTrials.gov (NCT04863352, on 27th of April 2021). All men provided written informed consent.
Statistical analysis
Characteristics were compared between men <70 and ≥70 years (of age) at treatment (date of RARP or first EBRT fraction) by independent samples t-test or χ2-test. The predefined primary outcome was global QoL as measured by QLQ-C30. Secondary outcomes were PF, measured by the QLQ-C30, and local side effects measured by the five domains of the EPIC-26.
We estimated a linear regression model to assess the association between the primary outcome, global QoL and age at treatment, a continuous variable (measured in years). Next, the model was adjusted for potential confounders, that is, treatment modality (RARP and EBRT), risk group (low/intermediate/high risk), clinical relapse (yes/no), time from treatment, cohabitant status (living alone/with others), education (four categories) and comorbidities (CCI-score). Treatment modality might potentially be an effect modifier for the association between age at treatment and outcome variables. Therefore, as planned a priori, interaction between non-linear age at treatment and treatment modality was included. The Bayes Information Criterion (BIC), where the smaller value means a better model, was applied to reduce the model for excessive non-linear terms and interactions. The same approach was applied to estimate linear regression models for the associations between the secondary outcomes and age at treatment. Potential non-linear association between global QoL and age was explored by including age as non-linear component (third-order polynomial). Potential non-linear associations between outcome and the continuous confounders (i.e. time from treatment and CCI-score) were assessed similarly.
To avoid uncertainties related to cancer relapse, we estimated similar regression models for men with no clinical relapse in sensitivity analyses. Residual diagnostics was performed by inspecting histograms and assessing heteroscedasticity graphically and multicollinearity through correlation analysis. No major deviations from the model assumptions were identified. Age at assessment and age at treatment were highly correlated, and only age at treatment was included in the models. No other multicollinearity issues were identified. All regression models were estimated for cases with no missing values on confounders.
Normative scores for QLQ-C30 were defined by assigning age-specific mean scores from a Norwegian general population [27] for each individual score. Since the normative population did not include men above 79 years, all men ≥70 years at treatment in our study population were assigned scores from the age group 70–79 years. The defined normative scores were then compared to observed QLQ-30 scores by paired-samples t-test. Results with p-values below 0.05 were considered statistically significant. All statistical analyses were performed in SPSS v27.
Results
A total of 888 men met the inclusion criteria, and 654 (74%) consented to participate in the study and answered the questionnaire. The response rate was comparable for older (≥70 years at treatment) and younger (<70 years at treatment) men, 243/345 (70%) and 411/543 (76%), respectively. Demographic and medical characteristics of the men are presented in Table 1. Median (min-max) age at treatment was 68 (44–84) years. Five hundred sixteen (79%) were treated with RARP, and 138 (21%) received EBRT (Table 1). Of the latter, 128 (93%) also received ADT (neoadjuvant only or neoadjuvant and adjuvant). Mean (SD) time between treatment and answering the questionnaire was 56.9 (16.4) months.
| Characteristics | Total N = 654 | <70 years at treatment N = 411 | ≥70 years at treatment N = 243 | p-value |
| Age at treatment Median (min-max) Age at first assessment Median (min-max) Months since start of treatment Mean (SD) Co-habitant status, n (%) Living alone Living with others (included partner/spouse) Missing Educational attainment, n (%) Primary school High school Vocational education College/ University Missing |
68 (44–84) 72 (49–91) 56.9 (16.4) 102 (16) 535 (82) 17 (3) 102 (16) 160 (24) 170 (26) 207 (32) 15 (2) |
64 (44–69) 69 (49–76) 58.0 (15.4) 50 (12) 350 (85) 11 (3) 56 (14) 101 (25) 115 (28) 131 (32) 8 (2) |
73 (70–84) 77 (72–91) 55.0 (17.8) 52 (21) 185 (76) 6 (2) 46 (19) 59 (24) 55 (23) 76 (31) 7 (3) |
0.0261 0.0022 0.2162 |
| EAU risk group, n (%) Low risk Intermediate risk High risk Missing Clinical relapse, n (%) No Missing Primary treatment, n (%) EBRT RARP ADT, n (%)3 No ADT Neoadjuvant Neoadjuvant and adjuvant CCI Min, max Mean (SD) |
31 (5) 364 (56) 249 (38) 10 (2) 497 (76) 5 (1) 138 (21) 516 (79) 10 (7) 47 (34) 81 (79) 0, 7 0.8 (1.2) |
26 (6) 247 (60) 131 (32) 7 (2) 304 (74) 0 28 (7) 383 (93) 2 (7) 7 (25) 19 (68) 0, 7 0.7 (1.1) |
5 (2) 117 (48) 118 (49) 3 (1) 193 (79) 5 (2) 110 (45) 133 (55) 8 (7) 40 (36) 62 (56) 0, 6 1.1 (1.3) |
<0.0012 0.0392 <0.0012 0.5052 <0.0011 |
| SD: standard deviation; EAU: European Association of Urology; EBRT: external beam radiotherapy; ADT: androgen deprivation therapy; RARP: robotic-assisted radical prostatectomy; CCI: Charlson comorbidity index. | ||||
| 1Independent samples t-test; 2χ2-test; 3% of primary treatment EBRT. | ||||
QLQ-C30 and EPIC-26 scores for older (≥70 years) and younger men (<70 years) are presented in Table 2. The proportion of men with missing scores for the various QLQ-C30 domains varied from 1 to 2%, and for most EPIC-26 domains from 2 to 3%, and was comparable between older and younger men. For the EPIC-26 sexual domain, the proportion of men with missing scales was 35% for those <70 years and 62% for those ≥70 years. Mean global QoL scores were similar in the older and younger groups (74.6 and 74.1, respectively). We found no clinically significant difference (≥10 points) between the two groups on any of the other QLQ-C30-scales/items or EPIC scores (Table 2).
| <70 years at treatment mean (SD) | ≥70 years at treatment mean (SD) | |
| EORTC QLQ-C301 | ||
| Global QoL | 74.1 (21.1) | 74.6 (20.2) |
| Physical function | 88.8 (15.1) | 81.4 (20.8) |
| Role function | 85.0 (23.5) | 79.7 (26.9) |
| Emotional function | 88.7 (16.9) | 90.3 (14.9) |
| Social function | 81.1 (23.5) | 81.3 (25.0) |
| Cognitive function | 86.5 (16.3) | 82.6 (18.1) |
| Fatigue | 25.6 (23.2) | 30.1 (23.0) |
| Nausea/vomiting | 1.3 (4.9) | 1.9 (7.4) |
| Pain | 21.6 (25.0) | 23.3 (26.2) |
| Dyspnoea | 15.8 (24.0) | 21.7 (28.7) |
| Sleeping disturbances | 17.8 (25.4) | 18.8 (25.2) |
| Appetite loss | 4.1 (14.9) | 5.3 (16.7) |
| Constipation | 10.7 (20.7) | 17.9 (26.9) |
| Diarrhoea | 12.5 (22.8) | 14.0 (23.3) |
| Financial difficulties | 5.3 (15.6) | 2.5 (10.3) |
| EPIC-262 | ||
| Urinary incontinence | 77.0 (24.5) | 79.3 (20.9) |
| Urinary irritative/obstructive | 88.4 (14.0) | 85.9 (14.4) |
| Bowel | 89.6 (15.4) | 88.2 (16.1) |
| Sexual | 33.0 (24.6) | 26.9 (19.3) |
| Hormonal/vitality | 86.5 (16.5) | 86.5 (14.1) |
| EORTC QLQ-C30: European Organisation of Research and Treatment in Cancer Quality of life Questionnaire-C30; QoL: quality of life; EPIC-26: Expanded Prostate Cancer Index Composite; SD: standard deviation. | ||
| 1The proportion of men with missing scores for the various QLQ-C30 domains varied between 1-2%, and was comparable between older and younger men. | ||
| 2The proportion of men with missing scores for most EPIC-26 domains varied between 2-13%, and was comparable between older and younger men. For the EPIC-26 sexual domain, the proportion of men with missing scores was 35% for those <70 years and 62% for those ≥70 years. | ||
No statistically significant interactions between age at treatment and treatment modality were found in any of the regression models. Additionally, according to BIC, these interactions could be eliminated from the models as they did not improve the model fit. We consequently kept all our models without interaction terms.
We found no association between age at treatment and global QoL, neither in the unadjusted nor the adjusted models (Table 3). Age at treatment and later PF were negatively associated according to the unadjusted linear regression model (regression coefficient [RC] −0.61 [95% CI −0.82; −0.41]). This association did not remain significant in the adjusted model (Table 3).
| Dependent variables | Unadjusted models | Adjusted models1 | ||
| RC (95% CI) | p-value | RC (95% CI) | p-value | |
| EORTC QLQ-C30 | ||||
| Global QoL | 0.00 (−0.25; 0.25) | 0.995 | 0.28 (−0.01; 0.56) | 0.062 |
| Physical function | −0.61 (−0.82; −0.41) | <0.001* | −0.19 (−0.43; 0.04) | 0.103 |
| EPIC-26 domains | ||||
| Urinary incontinence | 0.16 (−0.13; 0.46) | 0.279 | −0.13 (−0.49; 0.22) | 0.456 |
| Urinary irritative/obstructive | −0.20 (−0.37; −0.02) | 0.030* | 0.03 (−0.18; 0.23) | 0.789 |
| Bowel | −0.01 (−0.21; 0.18) | 0.880 | 0.22 (−0.002; 0.44) | 0.052 |
| Sexual | −0.77 (−1.14; −0.40) | <0.001* | −0.77 (−1.19; −0.36) | <0.001* |
| Hormonal/vitality | 0.11 (−0.08; 0.30) | 0.270 | 0.30 (0.09; 0.52) | 0.006* |
| RC: regression coefficient; CI: confidence interval; EORTC QLQ-C30: European Organisation of Research and Treatment in Cancer Quality of life Questionnaire-C30; QoL: quality of life; EPIC-26: Expanded Prostate Cancer Index Composite. *Level of significance p<0.05 | ||||
| 1Confounders adjusted for in analysis were: Treatment modality (RARP and EBRT), prostate cancer risk group (low/intermediate/high risk), clinical relapse (yes/no), time from treatment, cohabitant status (living alone/with others), education (four categories), and comorbidities (CCI). | ||||
In the unadjusted linear models for the EPIC-26 domains, age at treatment was negatively associated with the urinary irritative/obstructive domain (RC −0.20 [95% CI −0.37; −0.02]) and with the sexual domain (RC −0.77 [95% CI −1.14; −0.40]). In the adjusted linear models, only the association between age at treatment and the sexual domain remained significant (RC −0.77 [95% CI −1.19; −0.36]). In addition, there was a positive linear association between age at treatment and the vitality/hormonal domain (RC 0.30 [95% CI 0.09; 0.52]) (Table 3).
The sensitivity analysis, which excluded men with clinical relapse of prostate cancer, confirmed the findings of no negative impact of age (data not shown).
Normative scores
In comparison to normative QLQ-C30 scores, both the older and younger cohort reported statistically significantly worse global QoL, social function, pain, fatigue and constipation, statistically significantly better emotional function and less nausea and vomiting. The younger group also reported better cognitive function and less dyspnoea. None of the differences were clinically significant (Figure 1).
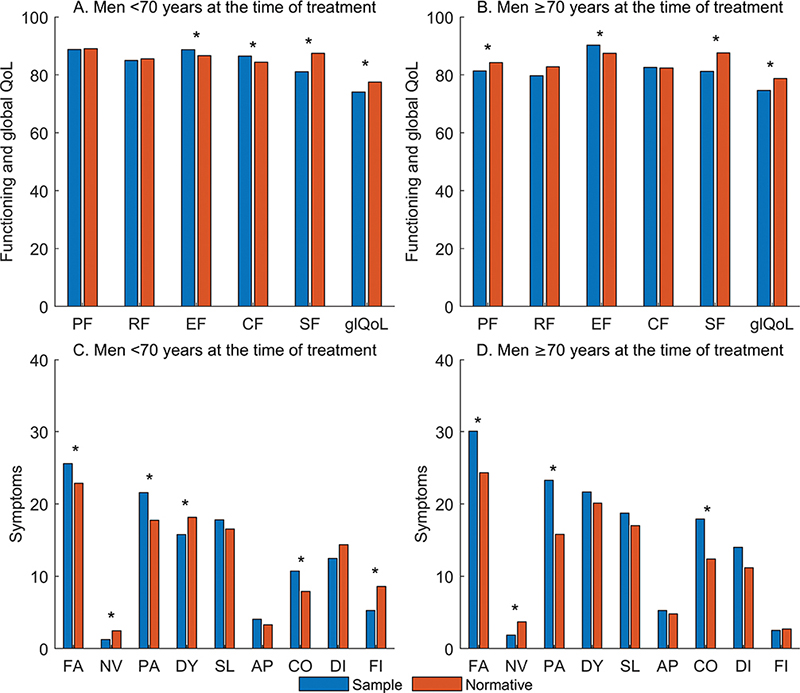
Figure 1. Comparison of QLQ-C30 functioning and global QoL sample scores to normative scores in two groups, men who were <70 years versus ≥70 years at the time of treatment.
PF: physical function; RF: role function; EF: emotional function; CF: cognitive function; SF: social function; glQoL: global QoL; FA: fatigue; NV: nausea/vomiting; PA: pain; DY: dyspnoea; SL: insomnia; AP: appetite loss; CO: constipation; DI: diarrhoea; FI: financial difficulties.
*indicates statistically significant differences, p < 0.05; no difference is clinically significant, that is, ≥10 points.
Discussion
In this cross-sectional study of men with prostate cancer receiving curative radiotherapy or surgery, we found no association between age at treatment and global QoL or PF 2–7 years later. Except for sexual function, age at treatment did not have a negative impact on local side effects and symptoms. For all QoL dimensions, there was no clinically significant difference between older and younger men, and there was no clinically significant deviance from normative population scores for either group.
In contrast to the majority of studies including PRO in curative treatment for prostate cancer, we chose to focus primarily on global QoL and PF, not treatment-related local side effects [2, 12]. Few studies have investigated the impact of age on such outcomes [12, 17]. Our findings are in line with a recent study reporting no significant difference between older (>70 years) and younger men on the SF-36 physical and mental summary scores 60 months after RARP [21]. They also partly agree with another study using the QLQ-C30 to assess QoL in Dutch men having received various treatments for a previously diagnosed prostate cancer [31]. No difference between older and younger men was found for most QLQ-C30 dimensions, but PF was lower for the older group, although not beyond 10 points [31]. Similarly, a longitudinal study found an association between older age at the time of RT and worse PF. However, illness perception and vitality were better, which corresponds to the positive association between age and hormonal/vitality scores found in the present study [32]. Taking studies including only older men into account, mainly stable QoL as measured by the QLQ-C30 and SF-36 after radical treatment is reported [18, 33], but declining functional independence has also been found [15]. Thus, results are not consistent, and comparison between studies is also hampered by differences in patient cohorts, study design, timing of assessment and assessment tools. However, joining present findings with previous ones, there are good indications that age at treatment has no major importance for general well-being and function after radical prostate cancer treatment.
PROs in terms of local side effects from radical prostate cancer treatment have been reported in several studies, and there seems to be an age-dependent relationship for some specific side effects. Increased risk of urinary incontinence after RARP seems to be related to higher age [16], but there are also studies that have different results [19, 20]. A recent review on radiotherapy for prostate cancer in older men [12] found acceptable tolerance with respect to urinary irritative symptoms and bowel toxicity, in line with our results. Our finding of a negative relationship between age and sexual dysfunction is in keeping with existing data [16], but the high proportion of men with missing scores in this particular domain makes it necessary to interpret our findings with caution. Recent studies also indicate that findings of poorer post-treatment scores in older men may be due to pre-treatment problems rather than treatment toxicity [21, 34].
In summary, QoL appears to be mainly good and preserved in older men after curative prostate cancer treatment. It must be noted that the men in this study had been carefully selected for treatment. The clinical selection process, including recommendation of either EBRT or RARP, is thus fundamental for our results, as is the case for most studies on QoL after prostate cancer treatment. That said, our findings render support to a growing understanding that other factors than chronological age may be the most important for tolerance of cancer treatment. There are several possible explanations to why QoL appears to be good. The results may be affected by difference in expectations between older and younger men. Expectations have a well-known impact on QoL [35]. In many aspects, older men may have lower expectations and consequently report better outcomes than younger ones in similar situations. Our data gives no insight into explanatory factors, but future research should explore this further.
Strengths and limitations
Strengths of our study are a relatively large study sample with a high proportion of older men, the use of well-validated QoL instruments, detailed information on prostate cancer treatment and comorbidity from electronic medical records, a high response rate and few missing values. Several limitations must also be considered. First, it is important to note the cross-sectional design and that data was collected on average about 4.5 years after the men had received their curative treatment. This means that our study gives no insight into the impact of age during the first months after treatment, or time-related changes. It is, for instance, possible that side-effects may have resolved over time, or that the men may have adapted to their situation, both resulting in improvement of QoL and a reduction of any difference to the general population. The cross-sectional design also implies that the time between treatment and QoL assessment varied. Although length of time after treatment was taken into account in our analyses, we cannot rule out that this could influence the results.
Moreover, we did not have pre-treatment data on the men’s QoL, function and symptoms, which hampers the evaluation of whether the radical treatment may have affected older and younger men differently. However, most problems assessed by our study questionnaires increase in frequency and severity with older age. It is unlikely that older men had significantly better pre-treatment scores than the younger ones, which would be the case if they experienced more severe declines and still ended up with scores comparable to their younger counterparts as found. Hence, we believe that the missing pre-treatment data do not affect our conclusions.
Second, our study cohort was heterogeneous in terms of treatment modality. Overall, it comprised a limited number of men receiving EBRT, in particular younger men, and in the group undergoing RARP, the number of older men were substantially smaller than the number of younger ones. Thus, related to differences in treatment modality, there are differences also in patients’ characteristics. Consequently, the impact of age may differ between modalities. We addressed this by interaction analyses, allowing us to preserve sample size. The interaction analyses did not show any statistically significant differences between treatment modalities with respect to association between age at treatment and outcomes. Despite this, and adjusting for treatment modality and other relevant confounders, we cannot rule out that the limited size of the group receiving EBRT and the skewness in age distribution may have influenced our results, in particular the results related to treatment-related side effects.
Third, our chosen cut-off of 70 years to define older or younger age might be seen as too low. However, compared to their younger counterparts, men ≥70 years with prostate cancer may be more susceptible to adverse effects [36] and, 70 years is the cut-off used to select men with prostate cancer in need of geriatric screening [1, 37]. Thus, we find that the cut-off was appropriate. In any case, the choice did not affect our main analyses where age was applied as continuous variable.
Finally, the results are based on a carefully selected population in clinical practice. Allocation to treatment modality was based on clinical judgement, including comorbidity and expected survival. Thus, our results cannot inform the choice between RARP or EBRT on older men with localized prostate cancer in general.
Conclusion
Our study adds insight into QoL in older men after curative treatment for prostate cancer. When treatment advice is individualized as in our cohort, later QoL seems to be good, and we found no large differences between older and younger men. Clinical decision-making should be based on biological age and not chronological age.
Acknowledgements
The authors are thankful to all former patients who participated in this study, and to study nurses Bodil Sem Kolsgaard and Anna Enger for their contribution in data collection.
Data availability statement
Due to a statement by the Data Protection Officer at Innlandet Hospital Trust, and in accordance with Norwegian privacy regulations, data cannot be shared publicly because they are confidential (due to the consent given by the men when included in the study). It is possible to extract information, upon request. Proposals should be directed to the Research Department of Innlandet Hospital Trust; contact: SIHFDLforskning@sikt.sykehuspartner.no.
ORCID
Reidun Sletten,  https://orcid.org/0009-0007-1192-2376
https://orcid.org/0009-0007-1192-2376
Ola Berger Christiansen,  https://orcid.org/0000-0003-1222-4207
https://orcid.org/0000-0003-1222-4207
Line Merethe Oldervoll,  https://orcid.org/0000-0002-5562-5396
https://orcid.org/0000-0002-5562-5396
Lennart Åstrøm,  https://orcid.org/0000-0003-1887-1392
https://orcid.org/0000-0003-1887-1392
Håvard Kjesbu Skjellegrind,  https://orcid.org/0000-0003-3067-0016
https://orcid.org/0000-0003-3067-0016
Jūratė Šaltytė Benth,  https://orcid.org/0000-0003-4199-2272
https://orcid.org/0000-0003-4199-2272
Øyvind Kirkevold,  https://orcid.org/0000-0001-5698-5636
https://orcid.org/0000-0001-5698-5636
Sverre Bergh,  https://orcid.org/0000-0001-9593-2967
https://orcid.org/0000-0001-9593-2967
Bjørn Henning Grønberg,  https://orcid.org/0000-0001-5744-1534
https://orcid.org/0000-0001-5744-1534
Siri Rostoft,  https://orcid.org/0000-0002-4277-8641
https://orcid.org/0000-0002-4277-8641
Asta Bye,  https://orcid.org/0000-0003-4902-0240
https://orcid.org/0000-0003-4902-0240
Paul Jarle Mork,  https://orcid.org/0000-0003-3355-2680
https://orcid.org/0000-0003-3355-2680
Marit Slaaen,  https://orcid.org/0000-0002-1038-8032
https://orcid.org/0000-0002-1038-8032
References
- [1] European Association of Urology: guidelines on Prostate Cancer 2023. [June 5th, 2023]. Available from: https://uroweb.org/guidelines/prostate-cancer
- [2] Lardas M, Liew M, van den Bergh RC, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol. 2017 Dec;72(6):869–885. https://doi.org/10.1016/j.eururo.2017.06.035
- [3] Fossa SD, Dahl AA, Johannesen TB, et al. Late adverse health outcomes and quality of life after curative radiotherapy + long-term ADT in prostate cancer survivors: comparison with men from the general population. Clin Transl Radiat Oncol. 2022 Nov;37:78–84. https://doi.org/10.1016/j.ctro.2022.08.003
- [4] Luo YH, Yang YW, Wu CF, et al. Fatigue prevalence in men treated for prostate cancer: a systematic review and meta-analysis. World J Clin Cases. 2021 Jul 26;9(21):5932–5942. https://doi.org/10.12998/wjcc.v9.i21.5932
- [5] Cancer Registry of Norway: Årsrapport 2021 med resultater og forbedringstiltak fra Nasjonalt kvalitetsregister for prostatakreft. Oslo; 2022 [cited 2023 Feb 13]. Available from: https://www.kreftregisteret.no/Generelt/Rapporter/Arsrapport-fra-kvalitetsregistrene/Arsrapport-fra-prostatacancerregisteret-/arsrapport-for-prostatakreft-2021/
- [6] Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer – 29-year follow-up. N Engl J Med. 2018 Dec 13;379(24):2319–2329. https://doi.org/10.1056/NEJMoa1807801
- [7] Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015 Mar 1;33(7):716–722. https://doi.org/10.1200/JCO.2014.57.2743
- [8] Aas K, Fossa SD, Åge Myklebust T, et al. Increased curative treatment is associated with decreased prostate cancer-specific and overall mortality in senior adults with high-risk prostate cancer; results from a national registry-based cohort study. Cancer Med. 2020 Sep;9(18):6646–6657. https://doi.org/10.1002/cam4.3297
- [9] Bratt O, Folkvaljon Y, Hjälm Eriksson M, et al. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur Urol. 2015 Jul;68(1):53–58. https://doi.org/10.1016/j.eururo.2014.12.026
- [10] Löffeler S, Halland A, Fawad H, et al. Non-metastatic prostate cancer: rationale for conservative treatment and impact on disease-related morbidity and mortality in the elderly. Scand J Urol. 2020 Apr;54(2):105–109. https://doi.org/10.1080/21681805.2020.1732463
- [11] Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021 Jan;71(1):78–92. https://doi.org/10.3322/caac.21638
- [12] Marotte D, Chand-Fouche ME, Boulahssass R, et al. Irradiation of localized prostate cancer in the elderly: a systematic literature review. Clin Transl Radiat Oncol. 2022 Jul;35:1–8. https://doi.org/10.1016/j.ctro.2022.04.006
- [13] Seghers P, Wiersma A, Festen S, et al. Patient preferences for treatment outcomes in oncology with a focus on the older patient – a systematic review. Cancers (Basel). 2022; 14(5): 1147. https://doi.org/10.3390/cancers14051147
- [14] Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002 Apr 4;346(14):1061–1066. https://doi.org/10.1056/NEJMsa012528
- [15] Ursem C, Diaz-Ramirez LG, Boscardin J, et al. Changes in functional status associated with radiation for prostate cancer in older veterans. J Geriatr Oncol. 2021;12(5):808–812. https://doi.org/10.1016/j.jgo.2020.12.011
- [16] Mandel P, Chandrasekar T, Chun FK, et al. Radical prostatectomy in patients aged 75 years or older: review of the literature. Asian J Androl. 2017 Sep 26;21(1):32–36. https://doi.org/10.4103/aja.aja_43_17
- [17] Odeo S, Degu A. Factors affecting health-related quality of life among prostate cancer patients: a systematic review. J Oncol Pharm Pract. 2020 Dec;26(8):1997–2010. https://doi.org/10.1177/1078155220959414
- [18] Goineau A, Campion L, Commer JM, et al. Can comprehensive geriatric assessment predict tolerance of radiotherapy for localized prostate cancer in men aged 75 years or older? Cancers (Basel). 2020 Mar;12:635. https://doi.org/10.3390/cancers12030635
- [19] Togashi K, Hatakeyama S, Okamoto T, et al. Oncologic and patient-reported outcomes after robot-assisted radical prostatectomy in men aged ≥75 years. Urol Oncol. 2021 Oct;39(10):729.e17–729.e25. https://doi.org/10.1016/j.urolonc.2020.12.001
- [20] Hampson LA, Cowan JE, Zhao S, et al. Impact of age on quality-of-life outcomes after treatment for localized prostate cancer. Eur Urol. 2015 Sep;68(3):480–486. https://doi.org/10.1016/j.eururo.2015.01.008
- [21] Posielski N, Frankel J, Kuo HC, et al. Impact of age and race on health-related quality of life outcomes in patients undergoing radical prostatectomy for localized prostate cancer. Urology. 2022 May;163:99–106. https://doi.org/10.1016/j.urology.2021.07.034
- [22] Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3;85(5):365–376. https://doi.org/10.1093/jnci/85.5.365
- [23] Szymanski KM, Wei JT, Dunn RL, et al. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010 Nov;76(5):1245–1250. https://doi.org/10.1016/j.urology.2010.01.027
- [24] Fossa SD, Storas AH, Steinsvik EA, et al. Psychometric testing of the Norwegian version of the Expanded Prostate Cancer Index Composite 26-item version (EPIC-26). Scand J Urology. 2016 Aug;50(4):280–285. https://doi.org/10.3109/21681805.2016.1163617
- [25] King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996 Dec;5(6):555–567. https://doi.org/10.1007/BF00439229
- [26] Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998 Jan;16(1):139–144. https://doi.org/10.1200/JCO.1998.16.1.139
- [27] Fossa SD, Hess SL, Dahl AA, et al. Stability of health-related quality of life in the Norwegian general population and impact of chronic morbidity in individuals with and without a cancer diagnosis. Acta Oncol. 2007;46(4):452–461. https://doi.org/10.1080/02841860601182641
- [28] Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015 Jan;85(1):101–105. https://doi.org/10.1016/j.urology.2014.08.044
- [29] Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004 Dec;57(12):1288–1294. https://doi.org/10.1016/j.jclinepi.2004.03.012
- [30] Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
- [31] Kurian CJ, Leader AE, Thong MSY, et al. Examining relationships between age at diagnosis and health-related quality of life outcomes in prostate cancer survivors. BMC Public Health. 2018 Aug 23;18(1):1060. https://doi.org/10.1186/s12889-018-5976-6
- [32] Lemanska A, Dearnaley DP, Jena R, et al. Older age, early symptoms and physical function are associated with the severity of late symptom clusters for men undergoing radiotherapy for prostate cancer. Clin Oncol (R Coll Radiol). 2018 Jun;30(6):334–345. https://doi.org/10.1016/j.clon.2018.01.016
- [33] Namiki S, Ishidoya S, Kawamura S, et al. Quality of life among elderly men treated for prostate cancer with either radical prostatectomy or external beam radiation therapy. J Cancer Res Clin Oncol. 2010 Mar;136(3):379–386. https://doi.org/10.1007/s00432-009-0665-6
- [34] Holze S, Bräunlich M, Mende M, et al. Age-stratified outcomes after radical prostatectomy in a randomized setting (LAP-01): do younger patients have more to lose? World J Urol. 2022;40(5):1151–1158. https://doi.org/10.1007/s00345-022-03945-0
- [35] World Health Organization – WHOQOL: measuring quality of life [December 4th, 2023]. Available from: https://www.who.int/tools/whoqol
- [36] Liang Y, Rausch C, Laflamme L, et al. Prevalence, trend and contributing factors of geriatric syndromes among older Swedes: results from the Stockholm County Council Public Health Surveys. BMC Geriatr. 2018 Dec 29;18(1):322. https://doi.org/10.1186/s12877-018-1018-6
- [37] Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019 Jul;116:116–136. https://doi.org/10.1016/j.ejca.2019.04.031