ORIGINAL RESEARCH ARTICLE
Prediction of clinically significant recurrence after partial nephrectomy. Data from the Cancer Registry of Norway with more than five years of follow-up
Ovidiu S. Barnoiua, Tom B. Johannesenb, Lien M. Diepc, Eskil S. Pederesena, Karin M. Hjelled and Christian Beislandd
aDepartment of Urology, Sorlandet Hospital, Kristiansand, Norway; bCancer Registry of Norway, Oslo, Norway; cOCBE, Research Support Services, Oslo University Hospital, Oslo, Norway; dDepartment of Urology, Haukeland University Hospital, Bergen, Norway
ABSTRACT
Objective: To determine recurrence incidence after partial nephrectomy (PN) for renal cell carcinoma and identify predictors for local recurrence (LR) and metastasis.
Material and methods: We retrospectively evaluated a cohort of 524 patients from the Cancer Registry of Norway, who underwent PN between January 2014 and December 2015 and were followed-up for >6 years. Patient demographics and pathological characteristics were correlated with recurrence and progression-free survival using Kaplan-Meier and Cox regression analyses.
Results: Median patient age was 64 years, and the median tumour size was 2.6 cm. A positive surgical margin (PSM) was observed in 11% of the cases, while the LR and metastasis rates were 3.4% and 3.2%, respectively. PSM (hazard ratio [HR], 55.4; 95% confidence interval [CI], 12.55–244.6), tumour number (HR, 45.4; 95% CI, 6.5–316.1) and stage (HR, 33.5; 95% CI, 5.4–205.3) were independent predictors for LR. Undetermined margin status was also a risk factor for LR. Tumour stage (HR, 41.05; 95% CI, 8.52–197.76), tumour necrosis (HR, 1.3; 95% CI, 0.4–4.31) and age (HR, 1.07; 95% CI, 1.01–1.14) were predictors for metastasis.
Conclusions: Both local and distant recurrences after PN were rare, and the pT stage was a common predictor. PSM or indeterminate surgical margin and tumour number were LR predictors, while age at surgery and the presence of tumour necrosis predicted metastasis.
KEYWORDS: Local recurrence; metastasis; partial nephrectomy; predictive model; renal cell carcinoma
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 1–9. https://doi.org/10.2340/sju.v59.18674.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 11 September 2023; Accepted: 5 December 2023; Published: 16 January 2024
CONTACT Ovidiu-Spiru Barnoiu ovidiu.spiru.barnoiu@gmail.com St. Olav’s vei 26, 301, NO-4631, Kristiansand, Norway.
Competing interests and funding: The authors report no conflicts of interest
This study was funded by our Institutional Research Department with an internal grant (number 21219094).
Introduction
Partial nephrectomy (PN) is the standard of care for patients with localised renal cell carcinoma (RCC) [1]. Nephron-sparing surgery should be performed if it does not compromise oncological, functional and perioperative outcomes. However, this approach is technically challenging and may yield positive surgical margins (PSMs) and disease recurrence.
Despite the ongoing debate concerning the role of PSMs in recurrence, the achievement of negative margins should be the main oncological concern in PN, and most systematic reviews have shown a higher risk of recurrence in patients with PSMs [2–4]. European guidelines recommend intensive follow-up of patients showing PSM for early detection of any recurrence [1] and recommend validated models, such as the Leibovich score for clear-cell RCC and the University of California Los Angeles integrated staging system for non-clear-cell RCC, to stratify the risk of recurrence after kidney surgery. However, these scores did not distinct the recurrence risk between patients operated with radical nephrectomy or PN. We believe that efforts should be made to separately predict recurrence in these two approaches, with an emphasis on PN.
Although recurrence is rarely observed after kidney surgery, the patterns and risk factors differ between radical nephrectomy and PN, both for low-stage [5] and more complex tumours [6, 7], and between local and distant recurrence [8]. This study aimed to assess the predictors of local and distant recurrence after PN using multicentric national registry data.
Material and methods
In Norway, by law, all new cases of cancer are required to be reported to the population-based Cancer Registry of Norway. The registry database employs a high-quality tool that sends reminders to various departments to submit missing reports; thus, the completeness rate is very close to 100% [9]. The registry did not include data on patients with benign tumours, and no patients with a proven benign histology after PN were registered in our study.
From the Cancer Registry of Norway, datasets for all 1,668 patients with RCC (ICD-10 code C64), from 2014 to 2015, were extracted from the primary database. Of these, 1,337 were surgically treated, and 543 patients who underwent PN remained within the dataset. Two authors manually performed quality assurance of all data used in the study, including re-evaluation of all histopathology reports. During this process, 19 patients with missing data for important variables were excluded (Figure 1). The final study population consisted of 524 patients, and their demographic, tumour-related, histology-related and 5-year follow-up data were transferred to an anonymised database for subsequent analysis.
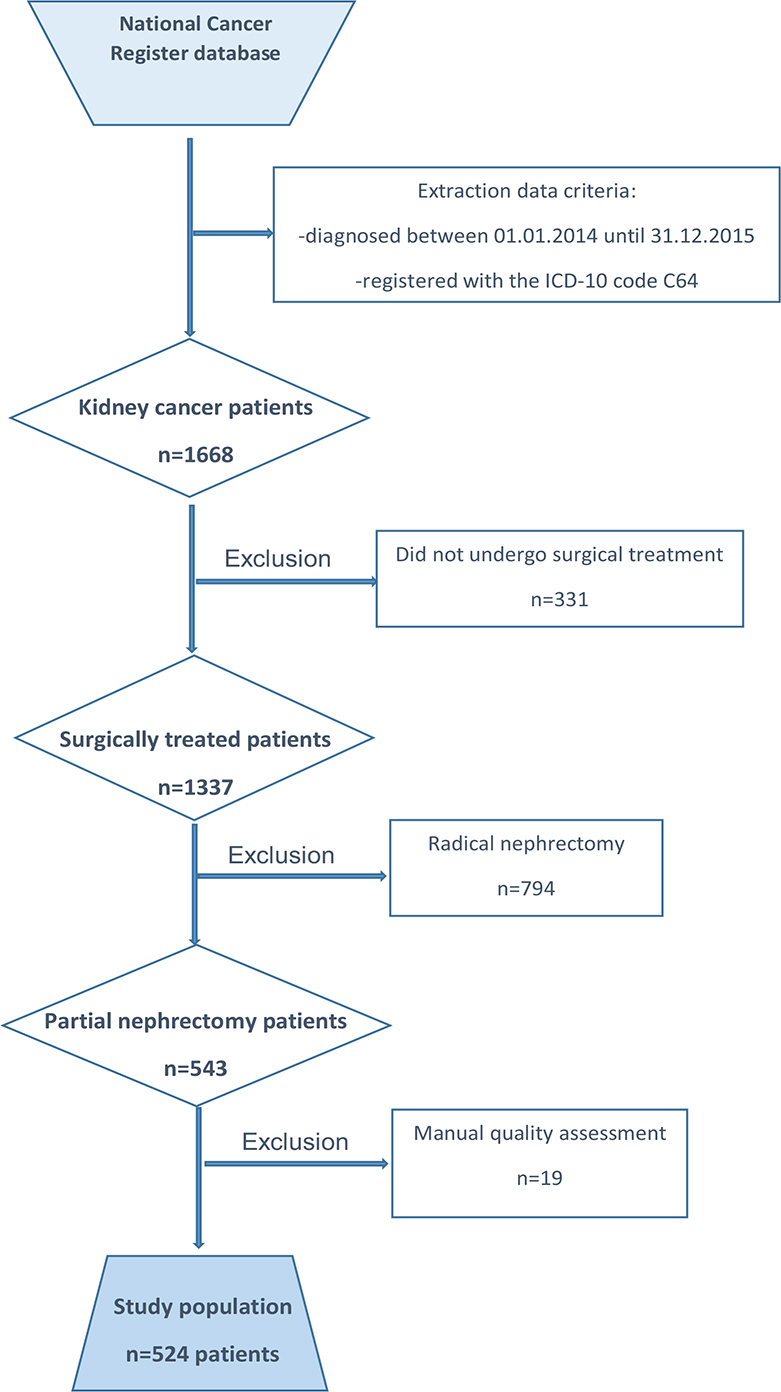
Figure 1. Flowchart for data extraction from the main database at the Cancer Registry of Norway according to the inclusion and exclusion criteria.
The analysed variables included age at diagnosis, sex, tumour characteristics, such as size and consistency (solid/cystic), number, surgery date and type (open/laparoscopy/robot-assisted), hospital volume, morphology type and grade, pT stage, the presence of tumour necrosis or capsule invasion, surgical margin status, the presence of local recurrence (LR) or metastasis (ME) and death date and cause. Since the Cancer Registry of Norway only includes histologically proven cancer, this study did not record recurrence or metastasis diagnosed clinically or on imaging but was not proven by biopsy. We defined so histologically proven recurrence as clinically significant for the patient’s prognosis, since a biopsy is mostly performed to decide the optimal management for patients who are candidates for active treatment.
The Cancer Register of Norway does not register data about the patient’s performance (e.g. Charlson comorbidity index, Eastern Cooperative Oncology Group performance status, previous surgery, solitary kidney, or body mass index) or nephrometry scores to assess tumour complexity. No patient had N+ stage disease or underwent lymphadenectomy concomitant with PN.
Hospital volume corresponding to each case was categorised as follows from the data for the number of procedures performed during the 2-year observation period: low volume, ≤10; intermediate volume, 11–20; high volume, 21–30; and very high volume, >30 procedures/year. The lower limit for the very-high-volume group was arbitrarily determined based on the presumed volumes at major academic hospitals in Norway. For analysis and presentation purposes, we grouped the pT stage and Fuhrman grade into three groups as follows: pT1a, pT1b and pT2 + pT3a and grades 1, 2 and 3 + 4, respectively. LR and ME were defined by the onset of neoplasms with identical histological patterns on the previous tumour resection bed or other organs, respectively.
Statistical analysis
Standard descriptive statistics were used for statistical analysis. Differences between groups were tested using the t test, Mann–Whitney–Wilcoxon test, chi-squared test, Fisher’s exact test, or Kruskal–Wallis test. An event was defined to have occurred if a patient developed LR or ME. The difference in survival between the two patient groups was tested using a log-rank test and illustrated using a Kaplan–Meier plot. Associations of patient characteristics and prognostic variables with LR and ME were assessed using Cox proportional-hazard regression. The strength of the association was quantified by determining the hazard ratio (HR) and 95% confidence interval (CI). P-values are provided for additional information. The proportional hazard assumption was examined graphically.
Results
Demographics
Table 1 summarises patient and tumour characteristics. The cohort consisted of 72% males (378 patients) with median age, 64 years (interquartile range [IQR], 54–79 years). Most tumours were solid (89%) and unifocal (97%; median [IQR] tumour size, 2.5 cm [1.8, 3.2 cm]). Of the surgeries, 42% were robot-assisted, 37% were laparoscopic and 13% were open, while more than three-fourths were performed in intermediate- (41%) and high-volume (27%) hospitals.
Histological features included a predominantly pT1a stage in almost 85% patients, clear cell type in nearly 70% of cases and Fuhrman grade 2 in 60% cases. Tumour necrosis and capsule infiltration were observed in 11% and 4% of patients, respectively.
Sixty cases (11%) showed PSM during the median (IQR) follow-up period of 81 months (75–88 months), with 18 patients (3.4%) showing LR and 17 patients (3.2%) showing ME. The median (IQR) time to LR was 35 months (24–56 months), which was 17 months longer than that to ME (median, 18 months; IQR, 13–42 months). Nearly 13% of the patients died during the follow-up period, and RCC was the cause of death in 18% of the patients.
Predictors for local recurrence
In the univariate analysis, PSM, tumour number, tumour size, pT stage and capsule infiltration showed significance for LR, but in multivariable Cox regression, only PSM (HR, 55.4; 95% CI, 12.55–244.6), tumour number (HR, 45.4; 95% CI, 6.5–316.1) and stage (HR, 33.5; 95% CI, 5.4–205.3) were independent predictors (Table 2). The LR-free survival was higher (Figure 2) in patients with negative margins (95% vs. 81% in those with PSM; Figure 2).
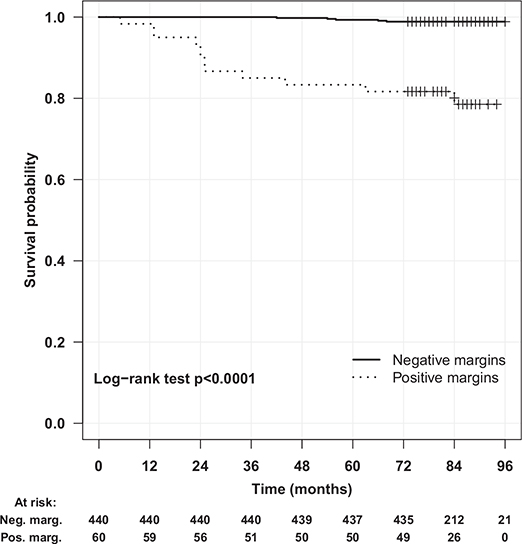
Figure 2. Local recurrence-free survival after partial nephrectomy.
Tumour size was excluded from the model because of its strong correlation with pT stage. The margin status after PN could not be definitively determined in 5% of the pathology reports, and Cox regression analysis showed that an undetermined margin status was also a risk factor for LR.
Predictors for metastasis
In the univariate analysis, age, tumour size, pT stage, nucleolar grade and the presence of necrosis were significant variables for ME, but in the multivariable Cox regression, only pT stage (HR, 41.05; 95% CI, 8.52–197.76), necrosis (HR, 5.24; 95% CI, 1.42–19.35) and age (HR, 1.07; 95% CI, 1.01–1.14) were independent predictors in the prediction model for ME (Table 3). PSM was not a risk factor for ME after PN. Tumour size was not included in the multivariate analysis because of its strong correlation with pT stage. ME-free survival was lower (Figure 3) in patients with tumour stage >pT2a (68% vs. 94% in patients with stage pT1a tumours).
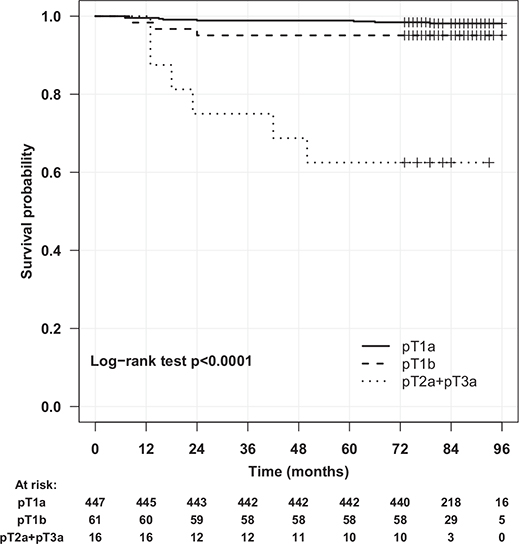
Figure 3. Metastasis-free survival after partial nephrectomy.
Discussion
In the data collected between 2014 and 2015, we found that local and distant recurrences after PN are rare. Metastasis occurred earlier than LR, and the pT stage was a common predictor of both local relapse and metastasis. A positive or indeterminate status of surgical margins and tumour number were predictors of LR, while age at surgery and the presence of tumour necrosis predicted metastasis.
Multiple series evaluating the recurrence after RCC surgery have been reported, and these studies have validated important prognostic factors such as tumour stage or the presence of coagulative necrosis. A main limitation of these studies was that they did not consider the type of surgery in assessments of recurrence, despite known differences in the pathological features associated with oncological outcomes between radical or PN. Moreover, few studies have evaluated recurrence risk after PN over a long follow-up period. Mouracade et al. showed that the features and risk factors of recurrence were different between patients showing LR and ME; however, their study had an intermediate follow-up, although the median time to recurrence in their study matched that reported in the literature [8]. We separately studied these two patterns of relapse and found low rates of LE and ME over a median follow-up period of 82 months. In our study, ME occurred earlier than LR (18 vs. 35 months), in contrast to the findings reported by Mouracade et al. (22 vs. 13 months), even though both studies showed similar time to ME. Wood et al. reported that the time to LR was 23 months [10], which was between our findings and those reported by Mouracade et al. A possible explanation could be the higher diagnostic rate of LR in previous studies and the fact that we only registered histologically proven recurrence since radiological recurrence was not registered in the Cancer Registry of Norway.
We assessed the prognostic factors previously related to recurrence and found that PSM, tumour number and stage were predictive of clinically significant LR, whereas age, tumour stage and the presence of tumour necrosis predicted the risk of ME. In particular, age was a strong predictor of overall survival after surgical treatment of RCC, as shown by Leibovich et al. [11]. In a recent study based on Swedish National Cancer Registry data, Almadalal et al. found that for both partial and radical nephrectomies, survival decreased in patients who developed ME, but the risk of LR did not increase with age [12]. In a study on recurrence after PN alone using data from a Canadian cancer registry, age was not predictive of progression, with a P-value of 0.06 close to statistical significance [13]. In our study, age was a predictor for ME but not LR, which may help navigate clinical decision-making before PN, especially in older patients, where the risk of non-RCC death exceeds the risk of recurrence due to cumulative comorbidities, as postulated by Stewart-Merrill et al. [14].
PSM was a risk factor for LR in both high-grade tumours, as shown by Shad et al. [15], similar to the pT1 stage, as demonstrated by Hendreickx et al. [3]. In addition, several meta-analyses have shown that PSM increases LR risk, and the latest such study by Garcia-Perdomo et al. [4] reported this finding with a moderate certainty of evidence. The same authors showed that PSM does not increase the risk of metastasis-free survival with a high certainty of evidence, as we found in our series. We recorded a high rate of PSM that may be related to beginning of the learning curve for robot surgery in some hospitals in Norway and also due to a low median tumour size in our series. Ficcara et al. [3] postulated that an inaccurate estimation of tumour extension as well as the absence or incomplete development of pseudocapsule and the accidental disintegration of the resection margins are more likely to occur in smaller tumours. In less than 5% of the patients in our series, the margin status could not be determined as positive or negative by the pathologist. This uncertainty in histological reporting of margin status was also proven to be a statistically significant risk factor for LR, and we believe that future studies should investigate if this feature could be considered, in practice, as a PSM.
Pathological T stage was expected to be predictive of recurrence because the disease was more aggressive, which agreed with previous studies [16]. Kim et al. also found that tumour stage was predictive of recurrence in patients with complex tumours, with a RENAL score >10 in the R domain (tumour size) being the main predictor of death [7]. A limitation of our study was the lack of assessments based on nephrometry scores, which were not registered in the cancer registry. Thus, we could only control for tumour size instead of the RENAL nephrometry score. The tumour size variable was excluded from our prediction model because of its strong correlation with pT stage, since the pathologic tumour stages <pT3 are defined by tumour size. Minervini et al. [16] found that a high tumour grade predicted long-term survival, especially for tumours >pT2, and Tagaki et al. [17] found that upstaging to pT3a together with a high grade was an independent factor for worse recurrence-free survival. They also included non-clear-cell RCCs as a predictive factor for recurrence after PN for T1a. In our study, both the histological subtype and nucleolar grade were found to be predictive of recurrence without any clinical explanation. In our study, tumour necrosis, which reflected aggressive biological activity, was a predictive factor for ME, in agreement with the literature [18]. In addition, capsule infiltration and sarcomatoid or rhabdoid differentiation were not included in the analysis because of the high rate of missing information in the histological reports.
Multifocal tumours at PN did not affect metastasis-free survival in a series of patients with hereditary RCC analysed by Gupta et al. over an intermediate follow-up without obtaining data regarding the effect on LR, which is impractical to assess in this group of patients [19]. Shah et al. did not find tumour number to be a risk factor for PSM [15], and Mouracade et al. showed no effect on disease-free survival [8]. In our study, we found more than one tumour to be a predictive factor only for LR, with a lack of information about hereditary cancers. In a study on multifocal RCC, Sorbellini et al. found a prevalence of at least 6% and presented favourable arguments for performing PN in this group of patients, although they strongly advocated appropriate surgery for the local control of renal lesions with negative margins [20]. Since multiple renal tumours appear to increase LR risk, in our opinion, it is important to intensify follow-up after PN for patients with additional risk factors such as PSM or high pT stage.
Regarding the surgical approach, a propensity score-matched analysis by Tam et al. [21] suggested that minimally invasive surgery results in a decreased risk of recurrence, with the limitation of requiring longer follow-up in cases involving an open approach, whereas in a case-control study by Wood et al., surgery type was not predictive of recurrence [10]. In our study, no significant difference was found between the robotic, laparoscopic and open approaches, but a possible bias could have occurred because the type of surgery was unknown in almost 15% of the patients. We did not find hospital volume to be a predictive factor for recurrence, although lower PSM rates may be found in high-volume hospitals when performing robot-assisted PN, as suggested by Xia et al. in a national cancer database analysis [22].
Limitations and strengths
This study had several methodological limitations, such as missing some important variables such as nephrometry scores, complication rates, warm ischaemia time, type of surgical technique, change in estimated glomerular filtration rate and long-term cancer-specific survival, which together could not suggest the centralisation or decentralisation of PN. The missing data for the invasion of the perirenal fat in the histological report limited our possibility to assess fat invasion as pT3a. Our study could not assess the role of surgeon experience in predicting recurrence, since the database included many surgeons with different levels of experience and was impossible to categorise.
This study had some other limitations, including the inherent problems of retrospective studies, the lack of information regarding patient characteristics related to comorbidities or performance status and tumour characteristics related to the nephrometry score or incomplete pathological reports. Since only histology-generating treatments were registered in the Cancer Registry of Norway, information regarding the treatment modality in case of recurrence, pT0 nephrectomies/metastasectomy or other forms of treatment such as ablation or radiotherapy was not registered. However, we consider histologically proven recurrence to have clinical significance for the patient’s prognosis.
We believe that data from the Norwegian cancer register are of high quality and provide great opportunities for real-world assessment of cancer-related outcomes. Our comprehensive national register follows the unique set-up that is available in the Nordic countries as shown in other national dataset from Scandinavia [23–24]. These factors, found in our study to be predictive for recurrence, could facilitate the development of a risk stratification score for recurrence if larger cohorts of patients with longer follow-up are used.
In conclusion, local and distant recurrences after PN are rare, and metastasis occurs earlier than LR. Only the pT stage was a common predictor of both local relapse and metastasis. A positive or indeterminate status of surgical margins and tumour number are predictors of LR, while age at surgery and the presence of tumour necrosis predict metastasis. The margin status did not predict metastasis.
Acknowledgments
This study was approved by our Institutional Review Board and by Regional Committees for Medical Research Ethics in Norway. In accordance with national regulations, our study did not require informed consent from patients for data extraction from the Cancer Registry of Norway, as the inclusion in the register is mandatory.
ORCID
Ovidiu-Spiru Barnoiu  http://orcid.org/0000-0003-3347-1645
http://orcid.org/0000-0003-3347-1645
Tom Borge Johannesen  https://orcid.org/0000-0001-8633-1633
https://orcid.org/0000-0001-8633-1633
Lien My Diep  https://orcid.org/0000-0003-2086-4909
https://orcid.org/0000-0003-2086-4909
Karin Margrete Hjelle  https://orcid.org/0000-0003-0999-1798
https://orcid.org/0000-0003-0999-1798
Christian Beisland  https://orcid.org/0000-0002-3216-4937
https://orcid.org/0000-0002-3216-4937
References
- [1] Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022 Oct;82(4):399-410. https://doi.org/10.1016/j.eururo.2022.03.006
- [2] Ficarra V, Crestani A, Inferrera A, Zargar H, Mottrie A, Autorino R. Positive Surgical Margins After Partial Nephrectomy: A Systematic Review and Meta-Analysis of Comparative Studies. Kidney Cancer 2018 Jan; 2(2):133-145. https://doi.org/10.3233/KCA-180037
- [3] Henderickx MMEL, Baldew SV, Marconi L, van Dijk MD, van Etten-Jamaludin FS, Lagerveld BW et al. Surgical margins after partial nephrectomy as prognostic factor for the risk of local recurrence in pT1 RCC: a systematic review and narrative synthesis. WorldJ Urol. 2022 Sep;40(9):2169-2179. https://doi.org/10.1007/s00345-022-04016-0
- [4] García-Perdomo HA, Ribal Caparrós MJ, Alcaraz Asensio A, Vilaseca Cabo A. Effect of Positive Surgical Margins in Patients Who Undergo a Partial Nephrectomy Regarding Recurrence, Overall Survival, Recurrence/Progression-Free Survival, and Metastasis-Free Survival. A Systematic Review and Meta-Analysis. Clin Genitourin Cancer. 2022 Oct;20(5):459-472. https://doi.org/10.1016/j.clgc.2022.05.011
- [5] Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011 Apr;59(4):543-52. https://doi.org/10.1016/j.eururo.2010.12.013
- [6] Jang HA, Kim JW, Byun SS, Hong SH, Kim YJ, Park YH et al. Oncologic and Functional Outcomes after Partial Nephrectomy Versus Radical Nephrectomy in T1b Renal Cell Carcinoma: A Multicenter, Matched Case-Control Study in Korean Patients. Cancer Res Treat. 2016 Apr;48(2):612-20. https://doi.org/10.4143/crt.2014.122
- [7] Kim H, Kim JK, Ye C, Choi JH, Lee H, Oh JJ et al. Recurrence after radical and partial nephrectomy in high complex renal tumor using propensity score matched analysis. Sci Rep 11, 2919 (2021). https://doi.org/10.1038/s41598-021-82700-8
- [8] Mouracade P, Kara O, Maurice MJ, Dagenais J, Malkoc E, Nelson RJ et al. Patterns and Predictors of Recurrence after Partial Nephrectomy for Kidney Tumors. J Urol. 2017 Jun;197(6):1403-1409. https://doi.org/10.1016/j.juro.2016.12.046
- [9] Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009 May;45(7):1218-1231. https://doi.org/10.1016/j.ejca.2008.10.037
- [10] Wood EL, Adibi M, Wei Q, Brandt J, Zhang M, Tamboli P et al. Local Tumor Bed Recurrence Following Partial Nephrectomy in Patients with Small Renal Masses. J Urol. 2018 Feb;199(2):393-400. https://doi.org/10.1016/j.juro.2017.09.072
- [11] Leibovich BC, Lohse CM, Cheville JC, Zaid HB, Boorjian SA, Frank I et al. Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. Eur Urol. 2018 May;73(5):772-780. https://doi.org/10.1016/j.eururo.2018.01.005
- [12] Almdalal T, Sundqvist P, Harmenberg U, Hellström M, Lindskog M, Lindblad P et al. Clinical T1a Renal Cell Carcinoma, Not Always a Harmless Disease-A National Register Study. Eur Urol Open Sci. 2022 Apr 1;39:22-28. https://doi.org/10.1016/j.euros.2022.03.005
- [13] Bansal RK, Tanguay S, Finelli A, Rendon R, Moore RB, Breau RH et al. Positive surgical margins during partial nephrectomy for renal cell carcinoma: Results from Canadian Kidney Cancer information system (CKCis) collaborative. Can Urol Assoc J. 2017 Jun;11(6):182-187. https://doi.org/10.5489/cuaj.4264
- [14] Stewart-Merrill SB, Thompson RH, Boorjian SA, Psutka SP, Lohse CM, Cheville JC et al. Oncologic Surveillance After Surgical Resection for Renal Cell Carcinoma: A Novel Risk-Based Approach. J Clin Oncol. 2015 Dec 10;33(35):4151-7. https://doi.org/10.1200/JCO.2015.61.8009
- [15] Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA et al. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J Urol. 2016 Aug;196(2):327-34. https://doi.org/10.1016/j.juro.2016.02.075
- [16] Minervini A, Lilas L, Minervini R, Selli C. Prognostic value of nuclear grading in patients with intracapsular (pT1-pT2) renal cell carcinoma. Long-term analysis in 213 patients. Cancer. 2002 May 15;94(10):2590-5. https://doi.org/10.1002/cncr.10510
- [17] Takagi T, Fukuda H, Ishihara H, Yoshida K, Kondo T, Kobayashi H et al. Predictive factors for recurrence after complete metatasetomy in patients with metastatic renal cell carcinoma in the targeted therapy era. Urol Oncol. 2020 May;38(5):515-520. https://doi.org/10.1016/j.urolonc.2020.02.003
- [18] Ito K, Seguchi K, Shimazaki H, Takahashi E, Tasaki S, Kuroda K et al. Tumor necrosis is a strong predictor for recurrence in patients with pathological T1a renal cell carcinoma. Oncol Lett. 2015 Jan;9(1):125-130. https://doi.org/10.3892/ol.2014.2670
- [19] Gupta GN, Peterson J, Thakore KN, Pinto PA, Linehan WM, Bratslavsky G. Oncological outcomes of partial nephrectomy for multifocal renal cell carcinoma greater than 4 cm. J Urol. 2010 Jul;184(1):59-63. https://doi.org/10.1016/j.juro.2010.03.035
- [20] Sorbellini M, Bratslavsky G. Decreasing the indications for radical nephrectomy: a study of multifocal renal cell carcinoma. Front Oncol. 2012 Aug 6;2:84. https://doi.org/10.3389/fonc.2012.00084
- [21] Tam AW, Kutikov A, Winoker JS, Rosenzweig S, Waingankar N, Okhawere KE et al. Propensity-score matched oncological outcomes and patterns of recurrence following open and minimally-invasive partial nephrectomy for renal cell carcinoma. Urol Oncol. 2022 Mar;40(3):111.e19-111.e25. https://doi.org/10.1016/j.urolonc.2021.12.011
- [22] Xia L, Pulido JE, Chelluri RR, Strother MC, Taylor BL, Raman JD et al. Hospital volume and outcomes of robot-assisted partial nephrectomy. BJU Int. 2018 Jun;121(6):900-907. https://doi.org/10.1111/bju.14099
- [23] Åkerlund J, Sundqvist P, Ljungberg B, Lundstam S, Peeker R, Månsson M et al. Predictors for complication in renal cancer surgery: a national register study. Scand J Urol. 2023 Aug 21;58:38-45. https://doi.org/10.2340/sju.v58.12356
- [24] Almdalal T, Karlsson Rosenblad A, Kjellman A, Lindblad P, Lundstam S et al. Predictive characteristics for disease recurrence and overall survival in non-metastatic clinical T1 renal cell carcinoma - results from the National Swedish Kidney Cancer Register. Scand J Urol. 2023 Feb-Dec;57(1-6):67-74. https://doi.org/10.1080/21681805.2022.2154383