ORIGINAL RESEARCH ARTICLE
DaBlaCa-17: nationwide observational study in Denmark on survival before and after implementation of neoadjuvant chemotherapy prior to cystectomy for muscle-invasive bladder cancer
Stefanie Korsgaard Körnera,b, Thomas Dreyera,b, Andreas Carusc,d, Line Hammer Dohne, Ulla Nordström Joensenf,g, Gitte Wrist Lamh, Niels Viggo Jenseni, Knud Fabrind,j, Thor Knak Jensenk, Helle Pappotg,l, Mads Agerbækm and Jørgen Bjerggaard Jensena,b
aDepartment of Urology, Aarhus University Hospital, Aarhus, Denmark; bDepartment of Clinical Medicine, Aarhus University, Aarhus, Denmark; cDepartment of Oncology, Aalborg University Hospital, Aalborg, Denmark; dDepartment of Clinical Medicine, Aalborg University, Aalborg, Denmark; eDepartment of Oncology, Herlev and Gentofte University Hospital, Copenhagen, Denmark; fDepartment of Urology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark; gDepartment of Clinical Medicine, Copenhagen University, Copenhagen, Denmark; hDepartment of Urology, Herlev and Gentofte University Hospital, Copenhagen, Denmark; iDepartment of Oncology, Odense University Hospital, Odense, Denmark; jDepartment of Urology, Aalborg University Hospital, Aalborg, Denmark; kDepartment of Urology, Odense University Hospital, Odense, Denmark; lDepartment of Oncology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark; mDepartment of Oncology, Aarhus University Hospital, Aarhus, Denmark
ABSTRACT
Objective: To investigate the impact of neoadjuvant chemotherapy implementation with gemcitabine-cisplatin on survival outcomes for patients with muscle-invasive bladder cancer in Denmark.
Materials and Methods: Data were collected on all patients in Denmark undergoing radical cystectomy who were potential candidates for neoadjuvant chemotherapy from 2010 to 2015 (n = 851). A cohort before the implementation of neoadjuvant chemotherapy (Cohort 2010–12) was compared with a cohort after implementation (Cohort 2013–15). Patients in Cohort 2013–15 receiving neoadjuvant chemotherapy (+NAC, n = 213) were compared with patients in Cohort 2013–15 not receiving neoadjuvant chemotherapy (-NAC, n = 139). Pathological results after radical cystectomy and oncological outcomes were compared between the study cohorts. Overall survival, disease-free survival, and disease-specific survival were compared with Kaplan-Meier plots and with univariable and multivariable Cox regression. Kaplan-Meier estimates of overall survival were also performed separately for treating hospital and for pathological stage.
Results: Pathological T0 (pT0) was more frequent in patients who received neoadjuvant chemotherapy: 34% versus 18% when comparing Cohort 2013–15 with Cohort 2010–12 (p < 0.001), and 46% versus 16% in +NAC compared with -NAC (p < 0.001). Overall survival, disease-free survival, and disease-specific survival at 5 years after cystectomy were not improved in Cohort 2013–15 compared with Cohort 2010–12 with adjusted hazard ratios of 1.11 (95% confidence interval [CI]: 0.87–1.43), 1.02 (95% CI: 0.81–1.29), and 1.06 (95% CI: 0.80–1.41), respectively.
Conclusions: This observational study found no improved survival in a national cohort of patients with muscle-invasive bladder cancer undergoing radical cystectomy after implementation of NAC. However, reservations should be made regarding the study design and the true effect of NAC on survival outcomes.
KEYWORDS: Bladder cancer; cystectomy; oncological outcomes; muscle-invasive; neoadjuvant chemotherapy; survival.
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 39–46. https://doi.org/10.2340/sju.v59.24024.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 19 October 2023; Accepted: 24 November 2023; Accepted: 30 January 2024; Published: 26 February 2024
CONTACT Stefanie Korsgaard Körner stkoer@rm.dk Department of Urology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, C118, DK-8200 Aarhus N, Denmark
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v59.24024
Competing interests and funding: The authors have no conflicts of interest to declare.
Introduction
The gold standard for treatment of muscle-invasive bladder cancer (MIBC) is radical cystectomy. Based on randomized clinical trials, neoadjuvant chemotherapy (NAC) is recommended in high-risk patients [1–4]. However, the overall effect size of survival benefit from NAC has been found to be limited [5]. A large proportion of patients will not be able to receive NAC due to impaired renal function or other comorbidities [4, 6]. Until 2013, NAC was not recommended in Denmark, and chemotherapy was administered only to patients with metastatic MIBC. NAC with four cycles of gemcitabine-cisplatin (GC) for eligible patients with MIBC was implemented nationwide on precisely 1 January 2013 [7]. The eligibility criteria for NAC in Denmark are urothelial carcinoma including variant histology, tumor stage cT2-T4aN0M0, creatinine clearance ≥60 ml/min, Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, age ≤75 years, and no other comorbidities contraindicating chemotherapy (<grade 2 audiometric hearing loss, <grade 2 peripheral neuropathy, <New York Heart Association (NYHA) Class III) [8].
The positive effects on survival in pathological complete responders (pT0) are well known [9, 10]. In a Danish national registry-based study, rate of downstaging was higher in patients treated with NAC and radical cystectomy compared to patients treated with radical cystectomy alone [11]. The impact of the nationwide NAC implementation with GC on long-term survival outcomes has not previously been analyzed.
The impact was evaluated by comparing all Danish patients with MIBC who fulfilled the eligibility criteria for NAC before and after 1 January 2013. Due to the sharp cut-off date for the nationwide implementation of NAC, comparing patients before and after implementation of NAC can be viewed as a natural experiment study with real-world evidence [12].
Methods
Patients
We included patients with MIBC tumor stage cT2–T4aN0M0 who fulfilled the following criteria: urothelial carcinoma, muscle-invasive disease at transurethral resection of bladder tumor (TURBT) (cT2+), normal kidney function (estimated glomerular filtration rate (eGFR) ≥50 ml/min was chosen as cut-off as precise GFR measurement was not available in the first time period) and age ≤75 years. Patients with urothelial carcinoma and components of variant histology were also included. The study population was divided into two cohorts: 1) Cohort 2010–12 consisted of patients who underwent radical cystectomy in the time-period before implementation of NAC from 1 January 2010 to 31 December 2012 and 2) Cohort 2013–15 consisted of patients who underwent radical cystectomy after implementation of NAC from 1 January 2013 to 31 December 2015. The NAC regime was four cycles of GC in a 3-week schedule. No adjuvant therapy was used during the study period. Apart from the introduction of NAC, no changes were made in recommended preoperative work-up, postoperative follow-up regimens, or surgical procedure during radical cystectomy. Radical cystectomy was performed as standard procedure with extended lymphadenectomy performed to the aortic bifurcation at four centers and at the iliac artery crossing of the ureter at one center (Aalborg University Hospital). Urethrectomy was performed in case of suspected or confirmed tumor involvement. Removal of female internal genitalia was performed. Urinary diversion was based on patient and operator preferences.
Data collection
Data were collected retrospectively from individual review of electronic medical records (EMR) at each of the five sites in Denmark where radical cystectomy is performed. We obtained the following data: whether the patient had received at least one cycle of NAC prior to cystectomy, creatinine level prior to NAC (or prior to cystectomy if the patient did not receive NAC), pathological tumor stage (pT), and lymph node stage (pN) of cystectomy specimen. Type of NAC and number of cycles administered were not registered, but four cycles of GC were the only recommended treatment in Denmark throughout the period. NAC was discontinued for patients with severe adverse events or loss of kidney function. Glomerular filtration rate (eGFR, ml/min) was estimated from creatinine using the CKD-EPI equation [13]. If patients recurred, date of first recurrence (clinically or histologically verified), type of recurrence (‘local’, ‘lymph node metastasis’, or ‘organ metastasis’), and treatment of recurrence were registered (‘surgery’, ‘systemic chemotherapy’, ‘systemic immunotherapy’, ‘curative radiation therapy’, ‘palliative radiation therapy’, or ‘no treatment/best supportive care’). Vital status was registered as either ‘related to bladder cancer’, ‘not related to bladder cancer’, or ‘no available info (NA)’. Death was registered as related to bladder cancer death if the patient died with metastatic or recurrent disease with no other obvious cause of death. As the Danish EMRs are national and include treating and admitting departments for cancer patients, data on treatment of recurrences are complete.
Statistical analysis
Overall survival (OS) was defined as time from date of radical cystectomy to date of death from any cause. Disease-free survival (DFS) was defined as time from radical cystectomy to date of recurrence or death from any cause. Disease-specific survival (DSS) was defined as time from radical cystectomy to death related to bladder cancer. For patients still alive, the censoring date was 31 December 2020. The follow-up period was estimated from date of radical cystectomy to date of death or censoring date. Kaplan-Meier estimates of OS, DFS, and DSS were calculated to compare Cohort 2010–12 with Cohort 2013–15, and for stratifying patients from Cohort 2013–15 into patients who received NAC (+NAC) and patients who did not (-NAC). In addition, Kaplan-Meier estimates for OS were performed separately for pathological stage in the cohorts and for the five centers in Denmark performing radical cystectomy (Figure S2). Pearson’s Chi-squared test and Fisher’s exact test were used to compare categorical variables, and Wilcoxon rank sum test was used to compare continuous variables. Univariable and multivariable Cox regression models were used to estimate hazard ratio (HR) and adjusted HR with 95% confidence intervals (CIs). Adjustment was made for covariates gender, age at radical cystectomy, treating hospital, year of radical cystectomy, and creatinine prior to radical cystectomy or NAC for patients who underwent NAC. The proportional hazards assumption of the cox regression analyses were checked by using Schoenfeld residuals against the transformed time. To account for competing risk, cumulative incidence functions were performed by modeling the cause-specific hazard function. Gray’s test was used to evaluate the cause-specific cumulative incidence functions. Information on recurrence and cause of death after radical cystectomy was calculated within five years of follow-up for each individual. All confidence intervals were two-sided and a Z-score of ±1.96 was used to calculate confidence intervals. Statistical analyses were performed using RStudio version 4.3.0.1 (Boston, Massachusetts) [14]. The project was registered on the internal list of research projects in the Central Denmark Region approved by the Danish Data Protection Agency (1-16-02-574-20). The Danish Patient Safety Authority and the Regional Ethical Committees approved the collection of data from medical records (permissions no. 3-3013-2584/1 and 1-45-70-40-20). The study was conducted by the Danish Bladder Cancer Group (DaBlaCa).
Results
Study population
In total, 851 patients with MIBC at the time of TURBT underwent radical cystectomy in Denmark between 2010 and 2015. We excluded 108 patients due to age ≥76 years, creatinine prior to cystectomy >160 µmol/L, eGFR <50 ml/min, missing data, or other histological type than urothelial carcinoma. Thus, 743 patients were included in the analysis. In Cohort 2010–12, 391 patients underwent radical cystectomy compared with 352 patients in Cohort 2013–15 (Figure 1). In Cohort 2013–15, 213 patients received NAC (61%). Slightly higher creatinine and higher eGFR prior to cystectomy were observed in Cohort 2013-15 (Table 1).
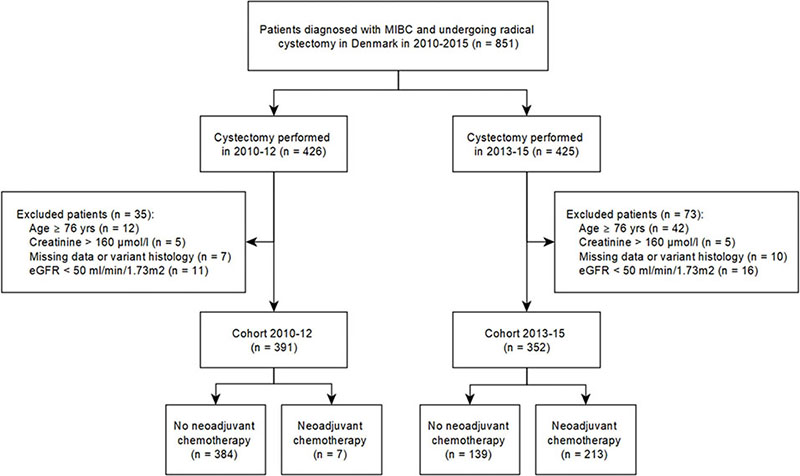
Figure 1. CONSORT-diagram of the study cohorts.
| Characteristic | N | Year of radical cystectomy | p-value2 | N | Radical cystectomy in 2013–15 | p-value2 | ||
| 2010–12, N = 391 | 2013–15, N = 352 | 2013–15 (+NAC), N = 213 | 2013–15 (-NAC), N = 139 | |||||
| Age at radical cystectomy1 | 743 | 65.8 (60.1–70.1) | 66.4 (60.6–71.1) | 0.2 | 352 | 65.3 (59.8–69.9) | 69.2 (62.2–72.6) | <0.001 |
| Follow-up, years1 | 743 | 8.3 (2.4–9.4) | 5.4 (1.4–6.6) | <0.001 | 352 | 5.5 (1.4–6.3) | 5.2 (1.3–6.8) | 0.6 |
| Gender | 743 | 0.7 | 352 | 0.2 | ||||
| Female | 94 (24%) | 91 (26%) | 50 (23%) | 41 (29%) | ||||
| Male | 296 (76%) | 261 (74%) | 163 (77%) | 98 (71%) | ||||
| NA | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Neoadjuvant chemotherapy | 743 | 7 (1.8%) | 213 (61%) | <0.001 | ||||
| Creatinine prior to cystectomy, µmol/L | 679 | 76 (66–86) | 82 (71–92) | <0.001 | 323 | 83 (73–91) | 79 (68–95) | 0.2 |
| Unknown | 35 | 29 | 12 | 17 | ||||
| eGFR, ml/min | 678 | 88 (75–95) | 82 (69–90) | <0.001 | 323 | 81 (70–88) | 83 (67–91) | >0.9 |
| Unknown | 36 | 29 | 12 | 17 | ||||
| T-stage at radical cystectomy | 743 | 352 | ||||||
| pT0 | 71 (18%) | 120 (34%) | <0.001 | 98 (46%) | 22 (16%) | <0.001 | ||
| pT1/CIS/pTa | 61 (16%) | 46 (13%) | 0.3 | 28 (13%) | 18 (13%) | >0.9 | ||
| pT2 | 98 (25%) | 61 (17%) | 0.010 | 31 (15%) | 30 (22%) | 0.089 | ||
| pT3/4 | 161 (41%) | 125 (36%) | 0.11 | 56 (26%) | 69 (50%) | <0.001 | ||
| N-stage at radical cystectomy | 743 | 352 | ||||||
| pN0 | 292 (75%) | 276 (78%) | 0.2 | 178 (84%) | 98 (71%) | 0.004 | ||
| pN1-3 | 96 (25%) | 72 (20%) | 0.2 | 32 (15%) | 40 (29%) | 0.002 | ||
| pNx | 2 (0.5%) | 2 (0.6%) | >0.9 | 1 (0.5%) | 1 (0.7%) | >0.9 | ||
| 1Median (25%-75%); n (%). 2Wilcoxon rank sum test; Fisher’s exact test; Pearson’s Chi-squared test. | ||||||||
Pathological outcome
After radical cystectomy, pT0 was more frequent in Cohort 2013–15 than in Cohort 2010–12 (34% vs. 18%) (Table 1). More patients in Cohort 2010–12 than in Cohort 2013–15 (25% vs. 17%) had muscle-invasive tumor pT2 in the cystectomy specimen. After stratification of Cohort 2013–15 according to NAC-treatment, pT0 was more frequent in +NAC patients than -NAC patients (46% vs. 16%) (Table 1). In Cohort 2010–12, pN1-3 was more frequent than in Cohort 2013-15 (25% vs. 20%). Furthermore, pN1-3 was less frequent in +NAC than -NAC in Cohort 2013–15 (15% vs. 29%).
Recurrence
Recurrence rates were the same for patients in Cohort 2010–12 and Cohort 2013–15 within 5 years of follow-up for each individual (35%). The average time to recurrence was 3 months longer in Cohort 2010–12 than in Cohort 2013–15 (Table 2). A slightly higher frequency of local recurrence and lymph node metastasis was observed in Cohort 2010–12 than in Cohort 2013–15 (40% vs. 35%), whereas a higher frequency of organ metastases was observed for Cohort 2013–15 (40% vs. 48%). When comparing +NAC patients and -NAC patients in Cohort 2013–15, we found a recurrence rate of 35% in both groups, and the difference in time to first recurrence was comparable with 7 months in +NAC patients and 6 months in -NAC patients. Furthermore, when comparing +NAC patients to -NAC patients of Cohort 2013–15, we found that organ metastasis was more frequent in +NAC patients (55% vs. 39% of patients with recurrence).
| Characteristic | N | Year of radical cystectomy | N | Radical cystectomy in 2013–15 | ||
| 2010–12, N = 391 | 2013–15, N = 352 | 2013–15 (+NAC), N = 213 | 2013–15 (-NAC), N = 139 | |||
| Recurrence | 260 | 136 (35%) | 124 (35%) | 124 | 75 (35%) | 49 (35%) |
| Time to first recurrence, months1 | 10 (5–19) | 7 (4–13) | 7 (4–13) | 6 (4–12) | ||
| Type of recurrence2 | ||||||
| Local | 55 (40%) | 44 (35%) | 24 (32%) | 20 (41%) | ||
| Lymph node metastasis | 23 (17%) | 17 (14%) | 9 (12%) | 8 (16%) | ||
| Organ metastasis | 55 (40%) | 60 (48%) | 41 (55%) | 19 (39%) | ||
| NA | 3 (2%) | 3 (2%) | 1 (1%) | 2 (4%) | ||
| Treatment of recurrence3 | 191 | 106 (78%) | 85 (69%) | 85 | 53 (%) | 32 (%) |
| Surgery | 10 (7%) | 11 (8%) | 5 (6%) | 6 (11%) | ||
| Systemic chemotherapy | 88 (59%) | 63 (44%) | 38 (42%) | 25 (47%) | ||
| Systemic immunotherapy | 1 (1%) | 2 (1%) | 1 (1%) | 1 (2%) | ||
| Curative radiation therapy | 2 (1%) | 3 (2%) | 1 (1%) | 2 (4%) | ||
| Palliative radiation therapy | 20 (13%) | 27 (19%) | 22 (24%) | 5 (9%) | ||
| Supportive care only | 29 (19%) | 37 (26%) | 23 (26%) | 14 (26%) | ||
| Cause of death | ||||||
| Not related to bladder cancer4 | 28 (19%) | 29 (20%) | 12 (14%) | 17 (27%) | ||
| Related to bladder cancer4 | 119 (81%) | 118 (80%) | 73 (86%) | 45 (73%) | ||
| Alive after 5 years of follow-up | 244 (62%) | 205 (58%) | 128 (60%) | 77 (55%) | ||
| 1Median (25%-75%); n (%). 2Percentage of patients with recurrence. 3Some patients received more than one treatment for recurrence. 4Percentage of total dead in each group. NAC = neoadjuvant chemotherapy. | ||||||
Survival analysis
Figure 2 shows the Kaplan-Meier curves of overall survival, disease-free survival, and disease-specific survival stratified according to treatment year. Table 3 shows the 5-year OS, DFS, and DSS for Cohort 2010–12 and Cohort 2013–15. Comparison of OS in Cohort 2010–12 versus Cohort 2013–15 showed a tendency towards lower OS in Cohort 2013–15 (HR: 1.19, 95% CI: 0.96–1.48). The tendency of lower OS (HR: 1.11, 95% CI: 0.87–1.43) was minimal after adjustment. Neither HRs nor adjusted HRs for DFS and DSS showed a significant difference between Cohort 2010–12 and Cohort 2013–15.
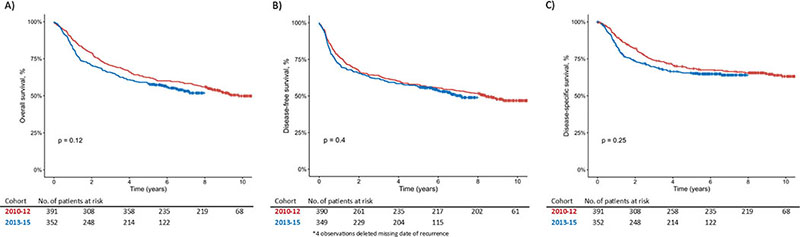
Figure 2. Kaplan-Meier curves of overall survival (A), disease-free survival (B), and disease-specific survival (C) stratified according to treatment year. Statistical test = log-rank-test. Censored observations marked as +.
| Year of radical cystectomy | Radical cystectomy in 2013–15 | |||||||
| 2010–12* | 2013–15 | HR (95% CI) | aHR (95% CI)1 | 2013–15 (+NAC) | 2013–15 (-NAC)* | HR (95% CI) | aHR (95% CI)2 | |
| 5-year survival | 5-year survival | 5-year survival | 5-year survival | |||||
| OS | 62% | 58% | 1.19 (0.96–1.48) | 1.11 (0.87–1.43) | 60% | 55% | 0.89 (0.65–1.21) | 0.94 (0.65–1.35) |
| DFS | 58% | 56% | 1.09 (0.89–1.35) | 1.02 (0.81–1.29) | 59% | 52% | 0.86 (0.64–1.17) | 0.91 (0.64–1.29) |
| DSS | 68% | 66% | 1.16 (0.90–1.48) | 1.06 (0.80–1.41) | 65% | 66% | 1.03 (0.72–1.49) | 1.06 (0.69–1.62) |
| 1Adjusted for gender, age at radical cystectomy, treating hospital, and creatinine prior to radical cystectomy or neoadjuvant chemotherapy for patients who underwent neoadjuvant chemotherapy. 2Adjusted for gender, age at radical cystectomy, creatinine prior to radical cystectomy or neoadjuvant chemotherapy for patients who underwent neoadjuvant chemotherapy, and year of radical cystectomy. | ||||||||
| *Reference. | ||||||||
| OS = overall survival, DFS = Disease-free survival, DSS = Disease-specific survival, HR = hazard ratio of death, aHR = adjusted hazard ratio of death, CI = confidence interval. | ||||||||
Figure S1 shows Kaplan-Meier curves of overall survival, disease-free survival, and disease-specific survival stratified according to treatment and year of treatment. Table 3 shows the 5-year OS, DFS, and DSS for Cohort 2013–15 stratified for NAC treatment and HRs and adjusted HRs. When stratifying according to site of cystectomy, one hospital had higher OS in Cohort 2010–12, whereas another hospital had lower OS in Cohort 2010–12 (Figure S2).
Figure 3 shows the comparison of clinical outcomes of patients stratified by pathological stage. Kaplan-Meier curves show overall survival of Cohort 2010-12, Cohort 2013-15, Cohort 2013-15 (+NAC), and Cohort 2013-15(-NAC) (Figure 3A–D, respectively).
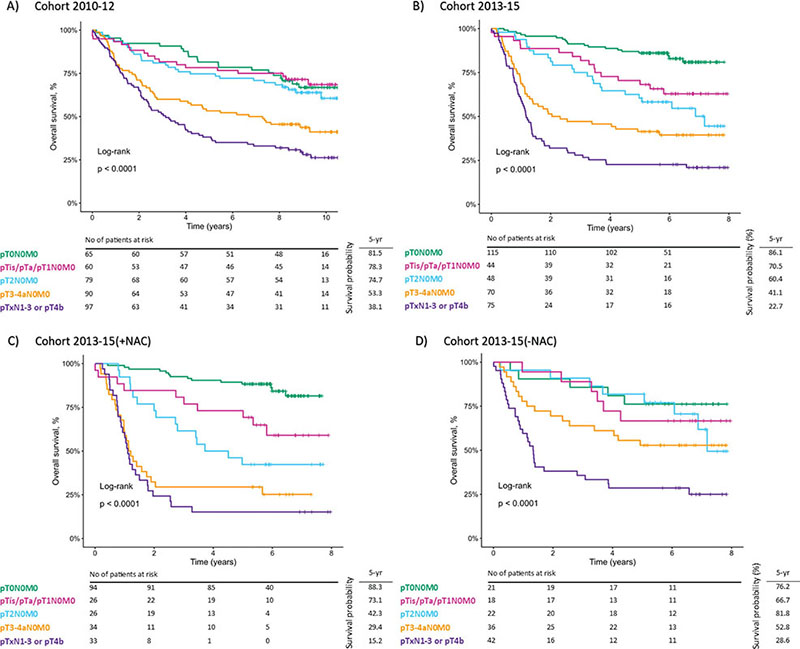
Figure 3. Comparison of clinical outcomes of patients with bladder cancer stratified by pathological stage. Kaplan-Meier curves show overall survival for (A) Cohort 2010–12, (B) Cohort 2013–15, (C) Cohort 2013–15(+NAC), and (D) Cohort 2013–15(-NAC) of patients with pT0N0M0 (green), pTis/pTa/pT1N0M0 (magenta), pT2N0M0 (aqua), pT3-4aN0M0 (orange), and pTxN1-3 or pT4b (purple). Statistical test = log-rank-test. Censored observations marked as +.
During the study period, 254 patients died of bladder cancer. Within 5 years of follow-up, 237 patients died of bladder cancer. In this follow-up period, death was related to bladder cancer in 81% of cases in Cohort 2010–12 and 80% of cases in Cohort 2013–15 (Table 2). Figure S3 models the cumulative incidence functions for bladder cancer death and other-cause mortality for both Cohort 2010–12 versus Cohort 2013–15 and for +NAC versus -NAC in Cohort 2013–15. We found no evidence to suggest that the cause-specific cumulative incidence curves for death from bladder cancer were not the same at all time points for Cohort 2010–12 versus Cohort 2013–15 and for +NAC versus -NAC in Cohort 2013–15. The same conclusion was made for other-cause mortality.
Discussion
In this national retrospective study, we found a higher percentage of complete local response (pT0) in the cystectomy specimens in Cohort 2013–15 than in Cohort 2010–12. However, the difference in pT0 did not translate into a difference in survival outcomes, and NAC was not associated with improved survival outcomes in either univariable or multivariable analyses. We found that MIBC patients have a poor prognosis, and for MIBC patients undergoing cystectomy in Denmark, OS does not seem to have improved after NAC implementation with GC.
This study aimed to evaluate the impact of nationwide implementation of NAC with GC on long-term oncological outcomes. The study is unique as all centers in Denmark implemented NAC at the same date and with the same criteria for patient selection from January 2013 and beyond. Assuming that MIBC patients fulfilling the aforementioned eligibility criteria before 1 January 2013 were not markedly different from patients after this date, these data provide a more robust analysis that is less prone to bias than purely observational studies [15].
The initial RCTs reporting a survival benefit from NAC included patients in the 1980s and 1990s, that is before modern imaging with PET/CT and extended lymph node dissection [1–3]. The exact role and potential benefit of PET/CT in diagnosis and staging of MIBC have yet to be fully investigated [4]. Extended lymph node dissection has previously been shown to improve OS [16]. Lymph node dissection to the level of the aortic bifurcation has previously been shown to be important for staging and prognosis [17]. However, the studies showing a survival benefit from lymph node dissection were conducted in patients who did not receive NAC. While we did not register the extent of lymph node dissection in the individual patients included in the present study, the template used in all participating sites remained constant throughout the study period.
A meta-analysis on NAC for MIBC found that overall survival increased by 8% over 5 years when comparing NAC to locoregional treatment alone [18]. However, only data extracted directly from published articles were available, and the studies were heterogeneous in terms of retrospective design, type of chemotherapy administered, and follow-up period. Moreover, the follow-up regimens are not uniformly clear [4]. Thus, in a modern patient cohort, where metastatic disease is more often detected with preoperative modern imaging and where extended lymph node dissection is performed, the potential of improving survival with NAC may therefore be lower than in the historical cohorts.
Population-based studies from the US, Sweden, and Norway have not found a significant survival benefit for patients receiving NAC compared with patients not receiving NAC [19–21]. A recent non-randomized study from Mitra et al. found that OS improved from 51% between 1990 and 1999 to 62% between 2010 and 2018, along with an increase in the use of NAC [22]. However, despite improved OS, neither NAC administration nor increased lymph node yield resulted in changes in recurrence-free survival (RFS). In a multivariable analysis, Mitra et al. found that both NAC and increased time from diagnosis to radical cystectomy increased the risk of recurrence. Furthermore, even after adjusting for causal relationships, NAC remained associated with lower OS and RFS. This indicates that not all patients will benefit from NAC and that postponing radical cystectomy to administer NAC is not beneficial for all patients. Møller et al. found a mediation effect of NAC on OS through downstaging even though they found no OS benefit of NAC [21]. They suggest that this could be explained by the NAC group including patients with more advanced or aggressive disease, and that some patients might not benefit from NAC. In future studies, efforts should be made to better identify patients who may benefit from NAC.
While clinical guidelines strongly recommend cisplatin-based NAC before radical cystectomy for patients with MIBC, the optimal treatment combination has yet to be determined [23]. In Denmark, the GC regimen is being used, whereas most previous RCTs have included methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) or similar regimens [1, 24]. Updated results from the randomized VESPER trial found a higher 5-year OS rate for patients treated with dose-dense MVAC (ddMVAC) than for patients who received GC in the neoadjuvant setting (66% vs. 57%) [25]. This suggests that ddMVAC could be the preferred treatment for NAC in MIBC. However, the ddMVAC regimen leads to a higher frequency of adverse events, in particular anemia, asthenia, and gastrointestinal symptoms [26]. Thus, perhaps more careful patient selection may be needed with ddMVAC to remedy this problem. The results from the VESPER trial regarding the GC arm may also explain why the present findings in the complete Danish cohort cannot replicate a clear survival benefit as found in earlier RCTs.
The results of the observational study are limited by a lack of information on performance status and comorbidities. Differences in patient selection for radical cystectomy between the two cohorts may exist for which we were unable to account. Clinical T-stage was inaccurately described in EMRs and therefore not included in the present study. Patients treated with NAC experience a delay to surgery. Since Danish patients are treated with four cycles of GC in a 3-week schedule, a minimum of a 12-week delay to surgery can be expected for patients completing four cycles. The design of the observational study causes some limitations. The first comparison is between two different time periods where 60% of patients in the late cohort received NAC. No information on the specific type of chemotherapy nor the number of completed NAC cycles was registered. Holmsten et al. found that 60% of the Danish study population received the planned four cycles of NAC and dose delay was reported in 27% of patients [27]. Another Danish study found that for 80% of patients who did not receive NAC in the period 2014–2017, the reason for omitting NAC was patients being unfit for cisplatin, while only 19% was due to patient refusal [28]. The second comparison performed was between +NAC and -NAC patients in Cohort 2013–15. A bias cannot be excluded, that patients with less aggressive cancers were less likely to receive NAC, which would also cause an underestimation of the potential true effect of NAC. Due to the study design, the potential true effect of NAC on survival outcomes will be underestimated.
In this nationwide observational study, we have presented survival outcomes in patients undergoing radical cystectomy for MIBC before and after nationwide implementation of NAC with GC. We observed no evidence of increased overall survival or disease-free survival after the implementation of NAC with GC. Reasons for these findings may include limited effect of NAC with GC in a modern MIBC patient cohort or differences in the cohorts that we were unable to account for. Due to the design of this observational study, reservations should be made for our results versus the true effect of NAC on survival outcomes.
Acknowledgements
The study was completed as part of a PhD study funded by The Danish Cancer Society [Grant no.: R231-A13800].
ORCID
Stefanie Korsgaard Körner,  https://orcid.org/0000-0002-6002-5037
https://orcid.org/0000-0002-6002-5037
References
- [1] Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. https://doi.org/10.1056/NEJMoa022148
- [2] Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45(3):297–303. https://doi.org/10.1016/j.eururo.2003.09.019
- [3] International Phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–2177. https://doi.org/10.1200/JCO.2010.32.3139
- [4] Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. https://doi.org/10.1016/j.eururo.2020.03.055
- [5] Nguyen DP, Thalmann GN. Contemporary update on neoadjuvant therapy for bladder cancer. Nat Rev Urol. 2017;14(6):348–358. https://doi.org/10.1038/nrurol.2017.30
- [6] Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘Unfit’ for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432–2438. https://doi.org/10.1200/JCO.2011.34.8433
- [7] DaBlaCa. Nationale kliniske retningslinier for behandling af blæretumorer i Danmark. [Cited 0 Nov. 2021] Available from: http://skejby.net/Webudgaven/Pdf/DaBlaCa_april_2016.pdf
- [8] DaBlaCa. Kliniske retningslinjer, behandling og opfølgning af muskelinvasiv blærekræft (in Danish) 2020 [updated 2021 Oct 29]. [Cited 14 Nov. 2023] Available from: https://www.dmcg.dk/siteassets/kliniske-retningslinjer---skabeloner-og-vejledninger/kliniske-retningslinjer-opdelt-pa-dmcg/blarecancer/dablaca_muskelinvasiv_1_1_admgodk111120.pdf
- [9] Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–1238. https://doi.org/10.1016/j.eururo.2011.12.010
- [10] Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65(2):350–357. https://doi.org/10.1016/j.eururo.2013.06.049
- [11] Körner SK, Jensen JB. A population-based retrospective analysis on variation in use of neoadjuvant chemotherapy depending on comorbidity in patients with muscle-invasive bladder cancer undergoing cystectomy in Denmark in the period 2013–2019. Scand J Urol. 2021; 56(1): 34–38. https://doi.org/10.1080/21681805.2021.2002400
- [12] Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551–555. https://doi.org/10.1038/bjc.2013.725
- [13] Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
- [14] R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
- [15] De Vocht F, Katikireddi SV, McQuire C, et al. Conceptualising natural and quasi experiments in public health. BMC Med Res Methodol. 2021;21(1):32. https://doi.org/10.1186/s12874-021-01224-x
- [16] Jensen JB, Ulhøi BP, Jensen KM-E. Extended versus limited lymph node dissection in radical cystectomy: impact on recurrence pattern and survival. Int J Urol. 2012;19(1):39–47. https://doi.org/10.1111/j.1442-2042.2011.02887.x
- [17] Nakagawa T. Lymph node dissection for bladder cancer: current standards and the latest evidence. Int J Urol. 2021;28(1):7–15. https://doi.org/10.1111/iju.14398
- [18] Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016;21(6):708–715. https://doi.org/10.1634/theoncologist.2015-0440
- [19] Hanna N, Trinh QD, Seisen T, et al. Effectiveness of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the current real world setting in the USA. Eur Urol Oncol. 2018;1(1):83–90. https://doi.org/10.1016/j.euo.2018.03.001
- [20] Russell B, Sherif A, Häggström C, et al. Neoadjuvant chemotherapy for muscle invasive bladder cancer: a nationwide investigation on survival. Scand J Urol. 2019;53(4):206–212. https://doi.org/10.1080/21681805.2019.1624611
- [21] Møller CT, Støer NC, Blindheim A, et al. Downstaging and survival after Neoadjuvant chemotherapy for bladder cancer in Norway; a population-based study. BMC Cancer. 2022;22(1):1301. https://doi.org/10.1186/s12885-022-10394-w
- [22] Mitra AP, Cai J, Miranda G, et al. Management trends and outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder: evolution of the University of Southern California experience over 3,347 cases. J Urol. 2022;207(2):302–313. https://doi.org/10.1097/JU.0000000000002242
- [23] Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021;79(1):82-104. https://doi.org/10.1016/j.eururo.2020.03.055
- [24] MRC/EORTC. Neoadjuvant cisplatin,methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet. 1999;354(9178):533–540. https://doi.org/10.1016/S0140-6736(99)02292-8
- [25] Pfister C, Gravis G, Flechon A, et al. Multicenter randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for muscle-invasive bladder cancer (MIBC): Overall survival (OS) data at 5 years in the GETUG/AFU V05 VESOER trial. JouJ Clin Oncol. 2023;41(17_suppl):LBA4507-LBA.
- [26] Pfister C, Gravis G, Fléchon A, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79(2):214–221. https://doi.org/10.1016/j.eururo.2020.08.024
- [27] Holmsten K, Omland L, Als A, et al. Implications for efficacy and safety of total dose and dose-intensity of neoadjuvant gemcitabine-cisplatin in muscle-invasive bladder cancer: three-week versus four-week regimen. Bladder Cancer. 2021;8(1):71–80. https://doi.org/10.3233/BLC-211556
- [28] Nielsen N, Wrist Lam G, Fabrin K, et al. Reasons why not all Danish patients with muscle invasive bladder cancer receive neoadjuvant chemotherapy before radical cystectomy. Scand J Urol. 2019;53(4):213–216. https://doi.org/10.1080/21681805.2019.1624608