ORIGINAL RESEARCH ARTICLE
Violation of onco-surgical principles is associated with survival outcomes in upper tract urothelial carcinomas after radical nephroureterectomy
Ioannis Patras, Johan Abrahamsson, Axel Gerdtsson, Martin Nyberg, Ymir Saemundsson, Elin Ståhl, Anne Sörenby, Åsa Warnolf, Johannes Bobjer and Fredrik Liedberg
Department of Urology Skåne University Hospital, Malmö, and Institution of Translational Medicine, Lund University, Malmö, Sweden
ABSTRACT
Objective: Disease recurrence, particularly intravesical recurrence (IVR) after radical nephroureterectomy (RNU) for upper tract urothelial carcinoma (UTUC), is common. We investigated whether violations of onco-surgical principles before or during RNU, collectively referred to as surgical violation (SV), were associated with survival outcomes.
Material and methods: Data from a consecutive series of patients who underwent RNU for UTUC 2001–2012 at Skåne University Hospital Lund/Malmö were collected. Preoperative insertion of a nephrostomy tube, opening the urinary tract during surgery or refraining from excising the distal ureter were considered as SVs. Survival outcomes in patients with and without SV (IVR-free [IVRFS], disease-specific [DSS] and overall survival [OS]) were assessed using multivariate Cox regression analyses (adjusted for tumour stage group, prior or concomitant bladder cancer, comorbidity and preoperative urinary cytology).
Results: Of 150 patients, 47 (31%) were subjected to at least one SV. Overall, SV was not associated with IVRFS (HR 0.81, 95% CI 0.4–1.6) but with worse DSS (HR 1.9, 95% CI 1.03–3.7) and OS (HR 1.9, 95% CI 1.2–3) in multivariable analysis. Additional analyses with a broader definition of SV including also preoperative instrumentation of the upper urinary tract (ureteroscopy and/or double J stenting) showed similar outcomes for DSS (HR 2.1, 95% CI 1.1–4.3).
Conclusion: Worse survival outcomes, despite no difference in IVR, for patients that were subjected to the violation of sound onco-surgical principles before or during RNU for UTUC strengthen the notion that adhering to such principles is a cornerstone in upper tract urothelial cancer surgery.
KEYWORDS: UTUC; Radical nephroureterectomy
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 131–136. https://doi.org/10.2340/sju.v59.25973.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 13 November 2023; Accepted: 27 May 2024; Published: 19 June 2024
CONTACT Ioannis Patras ioannis.patras@med.lu.se Department of Urology, Skåne University Hospital, Jan Waldenströms gata 5, SE-205 02 Malmö, Sweden
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v59.25973
Competing interests and funding: The authors have no relevant financial or non-financial interests to disclose.
This work was supported by the Swedish Cancer Society (CAN 2020/0709), Swedish Research Council (2021-00859), Lund Medical Faculty (ALF), Skåne University Hospital Research Funds, the Gyllenstierna Krapperup’s Foundation, The Cancer Research Fund at Malmö General Hospital, Skåne County Council’s Research and Development Foundation (REGSKANE-622351), Gösta Jönsson Research Foundation, the Foundation of Urological Research (Ove and Carin Carlsson bladder cancer donation) and Hillevi Fries Research. Foundation. The funding sources had no role in the study design, data analyses, interpretation of the results or writing of the manuscript.
Introduction
Disease recurrence including intravesical recurrence (IVR) after surgery for upper urothelial carcinoma (UTUC) is a common clinical problem after radical nephroureterectomy (RNU) [1]. Inadequate surgical techniques including violating sound onco-surgical principles (surgical violations [SVs]) such as intraoperative opening of the upper urinary tract during surgery or refraining from excising the distal ureter during RNU have recently been shown to increase the risk of disease recurrence [2]. Additionally, in selected patients, the diagnostic workup necessitates instrumentation of the upper urinary tract with ureteroscopy (URS), sometimes even with a biopsy, which increases the risk of IVR [1, 3, 4]. Furthermore, ureteric instrumentation such as URS and/or insertion of a double J stent or nephrostomy tube in a patient who subsequently is diagnosed with UTUC has also been associated with disease recurrence [5, 6].
The aim of this study was to evaluate the occurrence of SVs before or during RNU for UTUC and study associations between SV and intravesical recurrence-free survival (IVRFS), disease-specific survival (DSS) and overall survival (OS) at the long term. For this purpose, we investigated a consecutive cohort treated in a university hospital setting prior to centralisation of UTUC surgery in the Southern healthcare region of Sweden and prior to the first Swedish national urothelial carcinoma guidelines that were published in 2013 [7].
Material and methods
All 182 patients with suspected UTUC who underwent RNU between January 2001 and December 2012 at Skåne University Hospital, Malmö, Sweden were retrospectively identified. In 32 patients, the pathological report from the RNU specimen revealed non-urothelial cancer or benign findings were thus excluded (Figure 1).
Figure 1. CONSORT flowchart defining the study cohort.
Information about age, gender, American Society of Anaesthesiologists physical status (ASA-score) according to the American Society of Anaesthesiologists Classification [8], prior or concomitant bladder cancer, primary tumour location (renal pelvis, ureter or both), clinical TNM-groups, whether a preoperative URS and/or double J stent insertion was performed, outcomes from preoperative cytology (voided and selectively obtained) and surgical approach for RNU was retrieved from patient charts. Additionally, the following types of SVs were noted during chart review; intraoperative opening of the ureter during RNU, preoperative placement of a nephrostomy tube or intraoperative opening of the renal pelvis or leaving the distal ureter in situ without excising a bladder cuff during RNU.
No patients received perioperative chemotherapy. Following surgery, all patients were followed by cystoscopy and CT-urography, and disease status at the last follow-up was ascertained from patient charts for survival analyses.
Endpoints
Regarding IVRFS, the event was defined as the diagnosis of bladder cancer during subsequent follow-up. For DSS, the event was defined as death due to urothelial carcinoma, and for OS, the event was defined as death by any cause.
Statistical analyses
The proportions for each descriptive variable were compared between patients with and without SV by chi-square test and binomial logistic regression where appropriate. The study outcomes IVRFS, DSS and OS were visualised by Kaplan–Meier curves and compared by log-rank test. Associations with SVs were accomplished by applying multivariate Cox proportional hazards models to compare hazard ratios with a 95% confidence interval (CI) adjusting for the following confounders: ASA-score (1 vs. 2 vs. 3/4), prior or concomitant bladder cancer, clinical tumour stage group (stratified as Ta/T1 vs. T2–T4 and/or N+) and voided urinary cytology (benign vs. atypia/malignant). Survival estimates were calculated from the time of surgery to the time of event (IVR, death attributed to urothelial carcinoma or all-cause death, respectively). Given that preoperative invasive diagnostic modalities have been associated with the current study outcomes [9] additional analyses with a broader definition of SV were performed, including also preoperative instrumentation of the upper urinary tract as exposure.
All analyses were conducted by using the IBM® SPSS Statistics v.29.
This study was approved by the Research Ethics Board of Lund University, Sweden (EPN 2013/106 and 2013/4).
Results
Patient and treatment characteristics for the 150 patients are depicted in Table 1, where no differences were observed between the individuals in the two groups. The median follow-up time for patients without an event was 115 (interquartile range 73–144) months. In all, 61 SVs were registered in 47/150 (31%) of the patients. Among patients subjected to SVs, in 27/47 (57%) occasions, the ureter was opened intraoperatively, and in 12/47 (26%) patients, either a preoperative nephrostomy tube was inserted (6/47 [13%]), or the renal pelvis was opened intraoperatively (6/47 [13%]). In 22/47 (47%) patients, the distal ureter was left in situ during RNU.
| Baseline characteristics | Total | Surgical violation | ||
| Yes | No | |||
| n (%) | 150 | 47 (31) | 103 (69) | |
| Age, years | ||||
| Median (IQR) | 72 (64–78) | 74 (66–79) | 72 (64–78) | |
| Gender | Male | 86 | 20 (43) | 66 (64) |
| Female | 64 | 27 (57) | 37 (36) | |
| ASA | 1 | 23 | 6 (13) | 17 (17) |
| 2 | 86 | 27 (57) | 59 (57) | |
| 3 | 39 | 13 (28) | 26 (25) | |
| 4 | 2 | 1 (2) | 1 (1) | |
| Previous/concomitant bladder cancer | Yes | 30 | 7 (15) | 23 (22) |
| No | 120 | 40 (85) | 80 (78) | |
| Primary tumour location | Renal Pelvis | 98 | 36 (76) | 62 (60) |
| Ureter | 44 | 7 (15) | 37 (36) | |
| Both | 8 | 4 (9) | 4 (4) | |
| Clinical T stage* | Ta-T1 | 126 | 36 (76) | 90 (87) |
| T2–T4 | 24 | 11 (24) | 13 (13) | |
| Clinical N stage | N0 | 131 | 38 (37) | 93 (90) |
| N+ | 19 | 9 (63) | 10 (10) | |
| Ureteroscopy/JJ-stent prior to NU | Yes | 56 | 15 (32) | 41 (40) |
| No | 94 | 32 (68) | 62 (60) | |
| Voided urinary cytology | Not obtained | 28 | 6 (13) | 22 (21) |
| Benign | 35 | 15 (32) | 20 (20) | |
| Urinary atypia | 38 | 12 (26) | 26 (25) | |
| Malignant | 33 | 10 (21) | 23 (22) | |
| Malignant, high grade | 16 | 4 (8) | 12 (12) | |
| Selective urinary cytology | Not obtained | 120 | 41 (87) | 79 (76) |
| Benign | 11 | 2 (4) | 9 (9) | |
| Urinary atypia | 9 | 3 (6) | 6 (6) | |
| Malignant | 9 | 1 (1) | 8 (8) | |
| Malignant, high grade | 1 | 0 (0) | 1 (1) | |
| Year of surgery** | 2001–2003 | 17 | 8 (47) | 9 (53) |
| 2004–2006 | 37 | 7 (19) | 30 (81) | |
| 2007–2009 | 48 | 16 (33) | 32 (67) | |
| 2010–2012 | 48 | 16 (33) | 32 (67) | |
| Surgical approach | Open | 97 | 32 (68) | 65 (63) |
| Laparoscopic*** | 53 | 15 (32) | 38 (37) | |
| RNU: radical nephroureterectomy; IQR: Interquartile range. | ||||
| *Regarding distant metastases, there were two patients with a metastasis at the time of surgery, one undergoing a palliative nephrectomy and the other patient’s metastasis was discovered immediately after surgery, even though the patient underwent a preoperatively normal CT thorax. The first patient was included in the SV+ group while the second patient was in the SV group. | ||||
| **Percentage per row. | ||||
| ***Robot-assisted radical nephroureterectomy was performed in five patients. | ||||
During follow-up, IVR occurred in 14/47 (30%) patients with SV and 41/103 (40%) patients without SV. The corresponding rates of disease-specific death were 18/47 (38%) and 28/103 (27%) and for all-cause death 34/47 (72%) and 59/103 (57%), respectively. IVRFS, DSS and OS are visualised in Figure 2A–C. Multivariable models showed worse estimates of DSS (HR 1.9, 95% CI 1.0–3.7, P = 0.04) and OS (HR 1.9, 95% CI 1.2–3.1, P = 0.006) but not IVRFS (HR 0.81, 95% CI 0.4–1.6, P = 0.57) for patients subjected to SVs compared to patients without SV, as shown in Table 2 as well as in Supplemental Tables S1, S2 and S3.
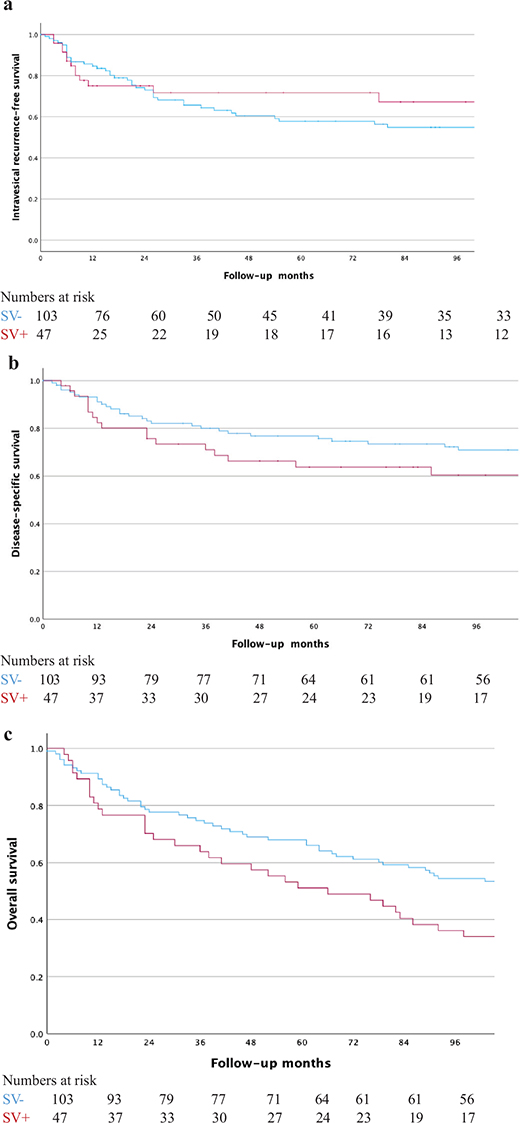
Figure 2. (A) Kaplan–Meier curves for intravesical recurrence-free survival stratified by surgical violation. (B) Kaplan–Meier curves for disease-specific stratified by surgical violation. (C). Kaplan–Meier curves for overall survival stratified by surgical violation.
With the broader definition of SV including also instrumentation of the upper urinary tracts, no associations with IVRFS (HR 1.5 95% CI 0.77–2.8, P = 0.24) or OS (HR 1.5, 95% CI 0.92–2.4, P = 0.10) were found, but the association with DSS remained when adjusting for the same covariates (HR 2.1, 95% CI 1.1–4.3, P = 0.036).
Discussion
In one of three patients (47/150, SV occurred in the form of intraoperative opening of the ureter/renal pelvis, preoperative placement of a nephrostomy tube and/or leaving the distal ureter in situ. Exposure to such SVs in conjunction with RNU was associated with both inferior DSS and OS with a follow-up of nearly 10 years. In contrast, intravesical recurrence-free survival (IVRFS) was not associated with SV.
The proportions of IVR in patients with and without SVs were 30% and 40% respectively, comparable to outcomes in a recent single-centre study reporting IVR rates of 48% [10] as well as IVR rates of 42% in the control arm of a randomised trial [10, 11]. Although invasive diagnostic modalities independently have been associated with IVR in population-based series [9] such association could not be confirmed in the current data even after additional analyses with preoperative instrumentation of the upper urinary tract included as exposure. The lack of information about other known risk factors for IVR such as tumour size, hydronephrosis and tumour multiplicity when performing time-to-event analysis [10] together with limited statistical power in the present study are possible explanations for the lack of association with IVRFS in the current study.
Several studies in the literature have demonstrated the pitfalls of incomplete resection of the upper urinary tract and in a series of 12 patients undergoing delayed completion ureterectomy, Abel et al. highlighted the importance of a complete en-bloc resection of the ureter to minimise the risk of both IVR and distant metastases [12] On the same theme, Carrion et al. reported local recurrences in 9 of the 21 patients in which the urinary tract was entered during laparoscopic RNU [13].
The rare and heterogenous nature of UTUC constitutes a challenge for the urologist both in planning the diagnostic work-up and in surgical treatment. To improve survival outcomes in patients undergoing RNU, quality of care indicators including a complete removal of the distal ureter with excision of a bladder cuff has been proposed as one of five measures to achieve pentafecta at RNU [14] Despite recommendations in guidelines, proportions without bladder cuff excision up to 30% in patients operated with RNU have been published [9, 15] In comparison, in the present study, 15% (22/150) of the patients were operated without bladder cuff excision. However, during later years in the authors’ institution, patients subjected to SVs tended to decrease, which might be partly due to increased surgical volumes over time [16]. This assumption is supported by a recently published study reporting an association between hospital volume and OS inferring that surgeon volume might contribute to this finding [17]. In line with this, SVs could be one possible mechanism behind the association between hospital volume and OS [17].
The current study is limited by the retrospective study design and reliance on chart review for the collection of the data. The small sample size also limits the modelling and number of covariates in time-to-event analysis, and residual confounding between the groups can thus not be excluded. However, the relatively high proportion of patients subjected to SVs increased the possibility to study this exposure despite the limited study population. Still, the sample size did not allow for an exploration of the impact of the different types of SVs included in the merged definition. Furthermore, the patients received their surgery prior to the introduction of new guidelines recommending postoperative bladder instillation of single-dose chemotherapy to decrease the risk of IVR. Thus, together with the infrequent use of lymph node dissection during RNU in the current series, the treatment regimen in the study is not complying completely with current quality indicators for RNU [18]. Additionally, applying the current level one evidence for adjuvant platinum-based chemotherapy for patients with pT2–pT4 and/or node-positive disease after RNU might have improved DSS in the current cohort and possibly even attenuated the detected differences between groups [19]. Yet, there could be a theoretical advantage of exploring the effects of SV in a population treated without perioperative chemotherapy, neither intravesical nor systemic.
One way to decrease the use of invasive diagnostic procedures and to increase compliance with current treatment guidelines has been suggested by streamlining the management of UTUC by means of creating a multidisciplinary tumour board (MDT) within the existing bladder cancer MDT [20]. By implementing such MDTs in a tertial referral centre in the UK, avoiding unnecessary diagnostic ureteroscopies and shortening time from referral to RNU was accomplished [20], in line with keeping surgical waiting times at a minimum given the reported association between longer delay than 1 or 2 months and oncological outcomes [21]. The high proportion of exclusions in the current study due to non-urothelial cancer or benign findings in the pathologic specimen (18%) decreased to a corresponding rate of only 2% in a more contemporary series of robotic RNU 2008–2021 in our institution, where the vast majority of patients were treated after the introduction of such MDTs [16].
Moreover, in September 2015, a standardszed care pathway (SCP) for patients with clinical suspicion of urothelial cancer has been implemented in Sweden with the ambition to reduce the time from first symptom to diagnosis but also to increase the quality of care and adherence to published guidelines [22]. Despite this, and the advent of adjuvant systemic treatment options after RNU that might decrease the risk of IVR [23] and/or at least partly compensate for the oncological hazards of SV, we believe that adequate surgery in patients with UTUC remains important. Further studies are needed to elucidate this, also in the context of current practice with postoperative intravesical instillations after RNU.
Conclusions
Applying sound onco-surgical principles when diagnosing and treating UTUC were associated with superior DSS and OS. To improve survival outcomes for this rare and poor prognosis disease, it seems reasonable to optimise the management to avoid SV, including a complete distal ureterectomy when performing RNU.
References
- [1] Rouprêt M, Seisen T, Birtle AJ, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 Update. Eur Urol. 2023;84(1):49–64. https://doi.org/10.1016/j.eururo.2023.03.013
- [2] Ryoo H, Kim J, Kim T, et al. Effects of complete bladder cuff removal on oncological outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Cancer Res Treat. 2021;53(3):795–802. https://doi.org/10.4143/crt.2020.919
- [3] Nowak Ł, Krajewski W, Chorbińska J, et al. The impact of diagnostic ureteroscopy prior to radical nephroureterectomy on oncological outcomes in patients with upper tract urothelial carcinoma: a comprehensive systematic review and meta-analysis. J Clin Med. 2021;10(18):4197. https://doi.org/10.3390/jcm10184197
- [4] Marchioni M, Primiceri G, Cindolo L, et al. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: a systematic review and meta-analysis. BJU Int. 2017;120(3):313–319. https://doi.org/10.1111/bju.13935
- [5] Schwartzmann I, Pastore AL, Saccà A, et al. Upper urinary tract urothelial carcinoma tumor seeding along percutaneous nephrostomy track: case report and review of the literature. Urol Int. 2017;98(1):115–119. https://doi.org/10.1159/000444808
- [6] Li YR, Yu KJ, Chang YH, et al. Predictors of intravesical recurrence after radical nephroureterectomy and prognosis in patients with upper tract urothelial carcinoma. Cancer Manag Res. 2020;12:7439–7450. https://doi.org/10.2147/CMAR.S261087
- [7] Liedberg F, Kjellström S, Lind AK, et al. Swedish National Guidelines on Urothelial Carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand J Urol. 2022;56(2):137–146. https://doi.org/10.1080/21681805.2022.2041086
- [8] Doyle DJ, Hendrix JM, Garmon EH. American Society of Anesthesiologists Classification. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [9] Liedberg F, Hagberg O, Häggström C, et al. Preoperative upper tract invasive diagnostic modalities are associated with intravesical recurrence following surgery for upper tract urothelial carcinoma: a population-based study. PLoS One. 2023;18(2):e0281304. https://doi.org/10.1371/journal.pone.0281304
- [10] Yonese I, Ito M, Waseda Y, et al. Impact of diagnostic ureteral catheterization on intravesical tumour recurrence following radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2023;41(7):1869–1875. https://doi.org/10.1007/s00345-023-04446-4
- [11] Ito A, Shintaku I, Satoh M, et al. Intravesical seeding of upper urinary tract urothelial carcinoma cells during nephroureterectomy: an exploratory analysis from the THPMG trial. Jpn J Clin Oncol. 2013;43(11):1139–1144. https://doi.org/10.1093/jjco/hyt129
- [12] Abel EJ, Fisher MB, Matin SF, et al. Delayed ureterectomy after incomplete nephroureterectomy for upper tract urothelial carcinoma: pathologic findings and outcomes. Int Braz J Urol. 2013;39(6):817–822. https://doi.org/10.1590/S1677-5538.IBJU.2013.06.07
- [13] Carrion A, Huguet J, García-Cruz E, et al. Intraoperative prognostic factors and atypical patterns of recurrence in patients with upper urinary tract urothelial carcinoma treated with laparoscopic radical nephroureterectomy. Scand J Urol. 2016;50(4):305–312. https://doi.org/10.3109/21681805.2016.1144219
- [14] König F, Grossmann NC, Soria F, et al. Pentafecta for radical nephroureterectomy in patients with high-risk upper tract urothelial carcinoma: a proposal for standardization of quality care metrics. Cancers (Basel). 2022;14(7):1781. https://doi.org/10.3390/cancers14071781
- [15] Nazzani S, Preisser F, Mazzone E, et al. Nephroureterectomy with or without bladder cuff excision for localized urothelial carcinoma of the renal pelvis. Eur Urol Focus. 2020;6(2):298–304. https://doi.org/10.1016/j.euf.2018.09.007
- [16] Liedberg F, Abrahamsson J, Bobjer J, et al. Robot-assisted nephroureterectomy for upper tract urothelial carcinoma-feasibility and complications: a single center experience. Scand J Urol. 2022;56(4):301–307. https://doi.org/10.1080/21681805.2022.2091018
- [17] Sui W, Wallis CJD, Luckenbaugh AN, et al. The impact of hospital volume on short-term and long-term outcomes for patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma. Urology. 2021;147:135–142. https://doi.org/10.1016/j.urology.2020.07.062
- [18] König F, Shariat SF, Karakiewicz PI, et al. Quality indicators for the management of high-risk upper tract urothelial carcinoma requiring radical nephroureterectomy. Curr Opin Urol. 2021;31(4):291–296. https://doi.org/10.1097/MOU.0000000000000895
- [19] Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–1277. https://doi.org/10.1016/S0140-6736(20)30415-3
- [20] Tay LJ, Chatterton K, Colemeadow J, et al. Improving management of upper tract urothelial carcinoma. BJU Int. 2020;126(1):5–6. https://doi.org/10.1111/bju.15068
- [21] Nowak Ł, Krajewski W, Łaszkiewicz J, et al. The impact of surgical waiting time on oncological outcomes in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy: a systematic review. J Clin Med. 2022;11(14):4007. https://doi.org/10.3390/jcm11144007
- [22] Abuhasanein S, Jahnson S, Aljabery F, et al. Standardized care pathways for patients with suspected urinary bladder cancer: the Swedish experience. Scand J Urol. 2022;56(3):227–232. https://doi.org/10.1080/21681805.2022.2058605
- [23] Yamaguchi N, Morizane S, Yumioka T, et al. Effect of adjuvant systemic chemotherapy on intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma. Anticancer Res. 2023;43(4):1725–1730. https://doi.org/10.21873/anticanres.16325