ORIGINAL RESEARCH ARTICLE
Normalised repeat serum prostate-specific antigen: associations with age and magnetic resonance imaging results
Hang Danga  , Victoria Huanga
, Victoria Huanga  and Ola Bratta,b
and Ola Bratta,b 
aDepartment of Urology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bDepartment of Urology, Sahlgrenska University Hospital, Gothenburg, Sweden
ABSTRACT
Objective: To assess the value of a repeat prostate-specific antigen measurement (PSA2) before magnetic resonance imaging (MRI) in men with a raised PSA (PSA1) <10 μg/L.
Method: Medical records of men aged < 75 years referred in 2021 for PSA1 3.0–9.9 μg/L (< 70 years) or 5.0–9.9 μg/L (70–74 years) were reviewed. PSA2 was sampled before MRI within 60 days from PSA1. Odds ratios (ORs) were calculated with logistic regression. Chi-square and trend-test were used for categorical variables.
Results: A total of 341 men were included. Median time between PSA1 and PSA2 was 28 days (interquartile range 20–35 days). PSA normalised in 16% (95% confidence interval [CI]: 13–21). Younger men were more likely to have a normal PSA2 (OR: 0.95 per year older, 95% CI: 0.92–0.99). Among men aged < 70 years, those with PSA1 < 5 μg/L were more likely to have normalised PSA2 than those with PSA1 ≥ 5 μg/L (21% vs. 10%, p = 0.01). A greater proportion of men with normalised PSA2 had a Prostate Imaging Data and Reporting System MRI score of 1–3 than men with non-normalised PSA2 (93% vs. 77%, p = 0.01).
Conclusions: A clinically significant proportion of men with a moderately raised PSA value have a normal PSA2. Younger men and men with lower PSA1 were more likely to have a normal PSA2. Few men with normalised PSA2 had suspicious MRI findings. Routine repeat PSA-testing may be motivated in men with a moderately raised PSA value to save MRI resources, particularly in younger men.
KEYWORDS: Prostate cancer; diagnosis; prostate-specific antigen; repeat testing; magnetic resonance imaging; age
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 54–57. https://doi.org/10.2340/sju.v59.26662.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 17 November 2023; Accepted: 19 January 2024; Published: 06 March 2024
CONTACT Hang Dang fudan1227@gmail.com Department of Urology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Introduction
Men with symptoms or findings suspicious of prostate cancer, such as a raised serum prostate-specific antigen (PSA) above the age-specific reference, are referred to a urologist for a diagnostic evaluation. In several countries, men with suspected prostate cancer will be referred according to a specific cancer patient pathway. The aims of cancer patient pathway programs are early detection of cancer and minimised time delay for diagnostics and treatments.
Serum PSA values may, however, fluctuate. Several previous studies assessed men with a moderately raised first PSA (PSA1) who had a repeat PSA (PSA2) sampled within 1–3 months before prostate biopsy [1–4]. PSA2 normalisation occurred in 11–25% of the men [2–4]. Of the men with PSA2 normalisation, 8–29% had prostate cancer and 3–5% had Gleason score ≥ 7 prostate cancer, whereas of those with non-normalised PSA2, 22–54% had prostate cancer and 12–34% had a Gleason score ≥ 7 cancer [2–4]. Furthermore, a PSA reduction of 20% or more was associated with a lower prostate cancer risk. The cancer risk reduction was greater in men younger than 60 years old [1].
The studies mentioned above were all based on a diagnostic pathway with a systematic biopsy without a pre-biopsy magnetic resonance imaging (MRI). However, most of the current prostate cancer diagnostic pathways include a pre-biopsy MRI and targeted biopsies because they reduce overdiagnosis of low-grade cancer and spare a considerable number of men to undergo a prostate biopsy [5].
The Swedish pathway includes a pre-biopsy MRI but did until the autumn of 2023 not include a repeat PSA test before the decision to book an MRI scan. The practice of referring all men with a single PSA value above the age-specific referral cut-off for an MRI scan put severe strain on the MR resources. The addition of a repeat PSA before the MRI scan might avoid unnecessary MRI scans, unnecessary prostate biopsies, and overdiagnosis of low-grade cancer. We therefore aimed at assessing the potential value of adding a repeat PSA before deciding whether to proceed with an MRI scan. Specifically, we analysed age, PSA levels, and MRI results in men with versus without PSA normalisation.
Methods
Study design and population
The study included men who in 2021 underwent investigation for prostate cancer according to the prostate cancer patient pathway at the Department of Urology, Sahlgrenska University Hospital, Sweden. Although the Swedish guidelines did not recommend a repeat PSA before booking an MRI scan they did recommend a repeat PSA before prostate biopsy. Therefore, when a patient started on the pathway both an MRI scan and a repeat PSA were booked. The repeat PSA was sampled just before or after the MRI scan, but the result of the repeat PSA test was not assessed until the urology appointment occurred.
The inclusion criteria were < 75 years old; PSA1 above the age-specific referral cut-off (≥ 3.0 μg/L for men aged < 70 years, ≥ 5.0 for men aged 70–80 years), and a PSA2 obtained before the MRI scan within 60 days after PSA1. Men whose PSA1 was ≥ 10 μg/L and those without PSA2 were excluded. The selection of the final study population is shown in Figure 1. The data were extracted from the electronic medical record program and its web applications. The study was approved by the Swedish Ethical Review Authority.
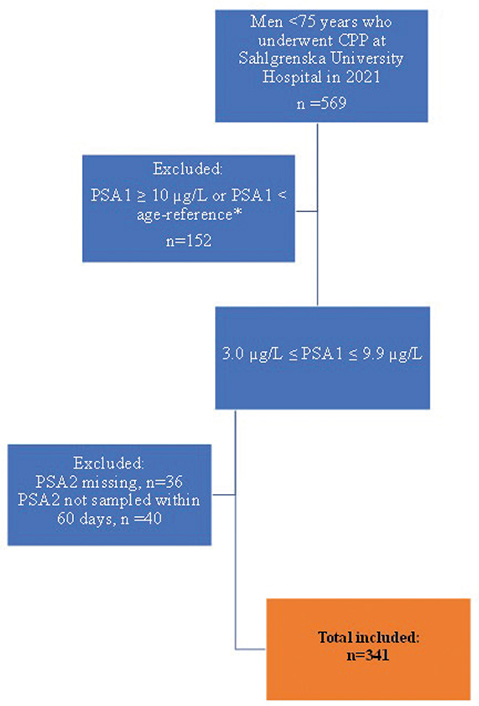
Figure 1. Flowchart for selection of the final study population.
CPP: Cancer Patient Pathway; PSA1: First prostate-specific antigen test; PSA2: Second prostate-specific antigen test. * PSA1 < 3 μg/L for men younger than 70 years or PSA1 < 5 μg/L for men 70 years or older.
Variables and outcome measures
PSA1 was defined as the first PSA obtained in connection with the cancer patient pathway program referral, while PSA2 was defined as the repeat PSA sampled nearest before MRI scan. If the date of PSA2 was not registered in the electronic medical record, the date was set as 3 days before the first urology appointment as this was the clinical routine.
MRI was assessed according to Prostate Imaging Reporting & Data System (PI-RADS) score. If several PI-RADS scores were available in the MRI report, the highest score was registered. The MRI result was considered non-interpretable if artefacts prevented assessment according to PI-RADS. According to the national guidelines, the indications for biopsy were suspicious digital rectal examination, PI-RADS 4-5 or PI-RADS 1-3 with PSA density ≥ 0.15 μg/L/cm3 [6]. If two or more biopsies were conducted in the cancer patient pathway (i.e. an immediate decision to re-biopsy was made based on the first biopsy result), the biopsy result with the highest Gleason score was chosen. PSA density (μg/L/cm3) for the men included in the study was calculated by using PSA1 and the prostate volume in the MRI report.
The primary outcome measure was the proportion of men with a PSA2 below the age-specific referral cut-off (for brevity, the term ‘normalised’ PSA is used in the text). Secondary outcome measures were the proportion of normalised PSA2 in men aged < 70 years with PSA1 < 5 μg/L versus ≥ 5 μg/L, the association between age and normalised PSA2, and MRI PI-RADS scores in men with normalised versus non-normalised PSA2.
Statistics
The statistical analyses were performed with IBM® SPSS® version 28. The Chi-square test was used to test PSA2 normalisation in men with different PI-RADS scores. To analyse the trend of PI-RADS score in correlation with PSA2 normalisation the Cochrane Armitage test was used, derived from linear-by-linear association in SPSS. Univariate logistic regression was employed to test the association between age and the normalised PSA2 for all men and in men < 70 years with PSA1 < 5 μg/L versus ≥ 5 μg/L.
Results
A total of 341 men were included in the analysis. The median value of PSA1 was 5.0 μg/L (Table 1). The median PSA2 was 4.8 μg/L for the total population, 2.6 μg/L for men with normalised PSA2 and 5.2 μg/L for men with non-normalised PSA2. The median time between PSA1 and PSA2 was 28 days for the entire population, 31 days for those with normalised PSA2 and 28 days in men with non-normalised PSA2.
| Variables | Total population n = 341 | Normalised PSA2 n = 55 | Non-normalised PSA2 n = 286 | |||
| Mean | Median (IQR) | Mean | Median (IQR) | Mean | Median (IQR) | |
| Age (years) | 63 | 64 (58–67) | 61 | 62 (55–67) | 63 | 64 (59–67) |
| PSA1 (μg/L) | 5.2 | 5.0 (3.7–6.5) | 4.6 | 3.7 (3.2–5.5) | 5.4 | 5.2 (3.9–6.6) |
| PSA2 (μg/L) | 5.2 | 4.8 (3.4–6.5) | 2.8 | 2.6 (1.9–2.8) | 5.7 | 5.2 (3.9–6.7) |
| Prostate volume (cm2) | 54 | 50 (36–64) | 45* | 43 (32–53) | 55** | 52 (38–66) |
| PSA–density (μg/L/cm3) | 0.11 | 0.10 (0.07–0.14) | 0.12* | 0.10 (0.07–0.14) | 0.11** | 0.10 (0.07–0.13) |
| Days between PSA1 and PSA2 | 28 | 28 (20–35) | 30 | 31 (25–37) | 27 | 28 (19–34) |
| * n = 54 (1 MRI scan was not conducted). ** n =275 (11 MRI scans were non-interpretable or not conducted). IQR: interquartile range; MRI: Magnetic Resonance Imaging; PSA1: First prostate-specific antigen test; PSA2: Second prostate-specific antigen test; Normalised PSA2: PSA < 3.0 μg/L for men < 70 years and < 5.0 μg/L for men 70–74 years. | ||||||
Proportions of men with a normal PSA2
Overall, 16% of the men had a normalised PSA2 (95% CI: 13–20). Younger men and men with a PSA1 < 5 μg/L were more likely to have normalised PSA2 (Figure 2). The odds ratio (OR) for normalised PSA2 was 0.95 per year increasing age (95% CI: 0.92–0.99).
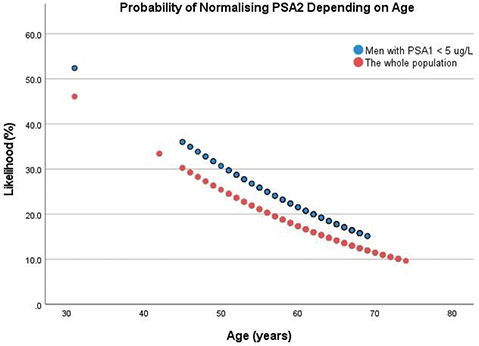
Figure 2. Likelihood (%) of a normal repeat PSA depending on age, calculated by univariate logistic regression.
For the whole population (n = 341): odds ratio = 0.95 (95% CI: 0.92–0.99, p = 0.02). For the group with PSA1 < 5 μg/L (n = 169): odds ratio = 0.95 (95% CI: 0.90–1.0, p = 0.08). CI: Confidence Interval; PSA1: First prostate-specific antigen test; PSA2: Second prostate-specific antigen test.
Among the 298 men who were younger than 70 years, 21% (95% CI: 15–28) with PSA1 < 5 μg/L and 10% (95% CI: 6.0–16) with PSA1 ≥ 5 μg/L (p = 0.01) had a normal PSA2.
At age 45–50 years, approximately 25–30% of the men had a normal PSA2, compared with approximately 10% of men aged 70–74 years (Figure 2). Among men aged 45–50 years with a PSA1 < 5 μg/L, the proportion with a normal PSA2 was 30–35%.
We did a subgroup analysis of men with a PSA2 sampled within 30 days after PSA1 (n = 205). In this subgroup 13% (95% CI: 8.9–18) had a normal PSA2. Of those who were younger than 70 years and had a PSA1 < 5 μg/L, 17% (95% CI: 11–26) had a normal PSA2. The corresponding proportion in those with PSA1 ≥ 5 μg/L was 6.7% (95% CI: 2.2–14).
MRI and biopsy findings in men with a normal versus a raised PSA2
Ten of the 341 men did not have an MRI scan for various reasons. The scan did not allow for PI-RADS scoring because of artifacts in three men, leaving 328 men for analysis of MRI results. One scan that was not PI-RADS scored did include a prostate volume measurement. Almost two-thirds of the men (65%) had PI-RADS 1-2. There was a significant negative association between the PI-RADS score and the proportion of men with normalised PSA2: the higher PI-RADS score, the lower proportion of men with normalised PSA2 (p = 0.003). A greater proportion of men with normalised PSA2 had PI-RADS 1-3 than men with non-normalised PSA2: 93% (95% CI: 82–97) versus 77% (95% CI: 72–82) (p = 0.01). None with normalised PSA2 had PI-RADS 5 (Table 2), while four of 54 (7.4%) had a PI-RADS 4 lesion.
Of the 312 men with a recorded digital rectal examination, 20 men had suspicious digital rectal examination. None of these 20 men had a normalised PSA2.
Only four of the 54 men with a normalised PSA2 had a biopsy, and they all had PI-RADS 4. Two had Gleason score 3 + 4 = 7 cancers with 10–20% pattern 4 (both MRI lesions 5 mm), one had Gleason score 3 + 3 = 6 cancer (lesion 5 mm), and one had a benign biopsy result (lesion 12 mm).
Discussion
We investigated the potential value of sampling a second PSA prior to MRI in a prostate cancer patient pathway and confirmed previous studies showing that a repeat PSA is often lower than the initial one [1–4]. The proportion of normalised PSA2 was 16%, which can be considered clinically relevant. We also found that younger men and those with PSA < 5 μg/L were more likely to have a normal PSA2. Men aged 50 years with PSA < 5 μg/L had more than 30% chance of having a PSA2 below 3 μg/L.
MRI findings in men with a normalised repeat PSA have not previously been reported. We observed a negative association between PI-RADS scores and the proportion of men with normalised PSA2. Most of the 54 men (82%) with normalised PSA2 had PI-RADS 1–2; only four out of the 54 men had a PI-RADS 4 lesion and no-one had a PI-RADS 5 lesion. In the four men with PI-RADS 4, biopsy showed favourable Gleason score 3 + 4 = 7 cancers in 2, and Gleason score 6 and benign tissue only in the other 2. It is thus unlikely that delaying the diagnosis in these men would have been detrimental.
A previous Swedish study reported a similar proportion (5.4%) of Gleason score ≥ 7 cancers that would not have been detected if biopsy had been omitted in men with PSA2 < 3 ng/mL [3]. A Northern Ireland study showed that of men with PSA normalised second PSA who later had a prostate biopsy, only 6.7% were diagnosed with a Gleason score ≥ 7 cancer after a mean follow-up of 5.9 year [7]. These studies, together with our own, suggest that omitting MRI in men who normalise their PSA value at repeat testing is associated with a low risk of missing cancer that require imminent radical treatment, although follow-up PSA testing seems prudent.
Our finding that PSA normalisation is particularly common in younger men agrees well with the results from the German screening study, in which as many as 48% of 45-year-old men had a PSA < 3 μg/L at a repeat PSA test 2 weeks after the first [8] and a recent Japanese study [9]. The latter study identified ejaculation and infection as possible causes of transiently rising PSA values.
One notable strength in our study is that the decision to undergo an MRI was independent of the results of PSA2, eliminating the risk of biases in selecting individuals for further diagnosis with MRI. However, it is important to acknowledge the limitations of this study. One weakness is the long and varying time between PSA1 and PSA2. Men with a short interval between PSA1 and PSA2 are probably less likely to normalise their PSA2 and it would not be feasible to routinely obtain a PSA2 after more than a few weeks. Moreover, our findings can only be applied to men of the same age (50–74 years) from a non-invited population (as opposed a population actively invited to an organised screening programme). Finally, patient selection into a prostate cancer diagnostic pathway may differ across countries and thereby the proportion of men with PSA normalisation on repeat PSA testing.
Conclusions
This study showed that a clinically significant proportion (16%) of men with an initially moderately raised PSA1 up to 9.9 μg/L in a cancer patient pathway had a normal PSA2 at repeat sampling after a median of 1 month. Younger men were more likely to have a normalised repeat PSA than older men, particularly those with a first PSA < 5 μg/L. Most men with a normalised PSA2 (82%) had unsuspicious MRI result and few were diagnosed with a Gleason score ≥ 7 cancer. Our findings support a routine with a repeat PSA after a few weeks before proceeding to an MRI scan in men with moderately raised PSA value, particularly in younger men with a first PSA < 5 μg/L. Nonetheless, prospective studies with larger sample sizes, a fixed time frame to the repeated PSA sample, and longer follow-up are needed to assess cost-effectiveness and the risk of unduly delaying the diagnosis of high-grade prostate cancer.
ORCID
Dang Hang  https://orcid.org/0009-0002-9707-9036
https://orcid.org/0009-0002-9707-9036
Huang Victoria  https://orcid.org/0009-0003-1276-8471
https://orcid.org/0009-0003-1276-8471
Bratt Ola  https://orcid.org/0000-0002-9198-9445
https://orcid.org/0000-0002-9198-9445
References
- [1] Rosario DJ, Lane JA, Metcalfe C, et al. Contribution of a single repeat PSA test to prostate cancer risk assessment: experience from the ProtecT study. Eur Urol. 2008;53(4):777–84. https://doi.org/10.1016/j.eururo.2007.11.064
- [2] De Nunzio C, Lombardo R, Nacchia A, et al. Repeat prostatespecific antigen (PSA) test before prostate biopsy: a 20% decrease in PSA values is associated with a reduced risk of cancer and particularly of high-grade cancer. BJU Int. 2018;122(1):83–8. https://doi.org/10.1111/bju.14197
- [3] Nordström T, Adolfsson J, Grönberg H, et al. Repeat prostate-specific antigen tests before prostate biopsy decisions. J Natl Cancer Inst. 2016;108(12):djw165. https://doi.org/10.1093/jnci/djw165
- [4] Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc. 2016;91(1):17–22. https://doi.org/10.1016/j.mayocp.2015.07.030
- [5] Xie J, Jin C, Liu M, et al. MRI/transrectal ultrasound fusion-guided targeted biopsy and transrectal ultrasound-guided systematic biopsy for diagnosis of prostate cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:880336. https://doi.org/10.3389/fonc.2022.880336
- [6] Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 1: early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand J Urol. 2022;56(4):265–73. https://doi.org/10.1080/21681805.2022.2094462
- [7] Connolly D, Black A, Murray LJ, et al. Repeating an abnormal prostate-specific antigen (PSA) level: how relevant is a decrease in PSA? Prostate Cancer Prostatic Dis. 2009;12(1):47–51. https://doi.org/10.1038/pcan.2008.37
- [8] Arsov C, Albers P, Herkommer K, et al. A randomized trial of risk-adapted screening for prostate cancer in young men – results of the first screening round of the PROBASE trial. Int J Cancer. 2022;150(11):1861–9. https://doi.org/10.1002/ijc.33940
- [9] Kobayashi M, Kijima T, Yashi M, et al. Prostate-specific antigen kinetics contributes to decision making for biopsy referral: the predictive implication for PSA retest in patients with elevated PSA levels. Prostate Int. 2023;11(1):27–33. https://doi.org/10.1016/j.prnil.2022.08.001