ORIGINAL RESEARCH ARTICLE
Temporal trend in risk of prostate cancer death in men with favourable-risk prostate cancer
Frederik F. Thomsena , Hans Garmob,c
, Hans Garmob,c , Lars Egevadd
, Lars Egevadd , Pär Stattinb
, Pär Stattinb and Klaus Brassoe,f
and Klaus Brassoe,f
aDepartment of Urology, Copenhagen University Hospital, Herlev and Gentofte Hospital, Herlev, Denmark; bDepartment of Surgical Sciences, Uppsala University Hospital, Uppsala, Sweden; cKing’s College London, School of Medicine, Division of Cancer Studies, Cancer Epidemiology Group, London, UK; dDepartment of Oncology-Pathology, Karolinska Institutet, Karolinska University Hospital, Solna, Stockholm, Sweden; eDepartment of Urology, Copenhagen Prostate Cancer Center, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark; fDepartment of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
ABSTRACT
Background and objectives: Changes in work-up and histopathological assessment have caused stage and grade migration in men with prostate cancer (PCa). The aim of this study was to assess temporal trends in risk of PCa death for men with favourable-risk PCa managed with primary radical prostatectomy or observation.
Methods and material: Men aged 75 or younger with Charlson Comorbidity index 0–1 diagnosed with favourable-risk PCa (T1–T2, prostate specific antigen [PSA] <20 ng/mL and Gleason score 6 or 7[3+4]) in the period 2000–2016 who were treated with primary radical prostatectomy or managed with observation in PCBaSe 4.0. Treatment groups were compared following propensity score matching, and risk of PCa death was estimated by use of Cox regression analyses.
Results: A total of 9,666 men were selected for each treatment strategy. The 7-year cumulative incidence of PCa death decreased in all risk and treatment groups. For example, the incidence in men diagnosed with low-risk PCa and managed with observation was 1.2% in 2000–2005, which decreased to 0.4% in 2011–2016. Corresponding incidences for men with intermediate-risk PCa managed with observation were 2.0% and 0.7%. The relative risk of PCa death was lower in men with low-risk PCa managed with radical prostatectomy compared to observation: in 2000–2005 hazard ratio (HR) 0.20 (95% confidence interval [CI] 0.10–0.38) and in 2011–2016 HR 0.35 (95% CI 0.05–2.26). Corresponding risks for men with intermediate-risk PCa were HR 0.28 (95% CI 0.16–0.47) and HR 0.21 (95% CI 0.04–1.18). The absolute risk reduction of radical prostatectomy compared to observation for men with low-risk PCa was 1% in 2000–2005 and 0.4% in 2011–2016, and for men with intermediate-risk PCa 1.1% in 2000–2005 and 0.7% in 2011–2016.
Conclusion: Men diagnosed in 2011–2016 with low-risk and favourable intermediate-risk PCa have a similar relative benefit but smaller absolute benefit of curative treatment compared to men diagnosed in 2000–2005.
KEYWORDS: Prostate cancer; radical prostatectomy; observation; watchful waiting; active surveillance; mortality; PCBaSe
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 76–83. https://doi.org/10.2340/sju.v59.34015.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 4 December 2023; Accepted: 11 April 2024; Published: 29 April 2024
CONTACT Frederik F. Thomsen thomsen.frederik@gmail.com Department of Urology, Copenhagen University Hospital, Herlev and Gentofte Hospital, Herlev, Denmark
Competing interests and funding: No author reports any conflict of interest.
This study was supported by The Swedish Cancer Society (190030) and Region Uppsala. These grants were unconditional, and the funding organisation had no influence on the work performed.
Introduction
Two randomised controlled trials (RCTs); Scandinavian Prostate Cancer Group 4 (SPCG-4) and Prostate Cancer Intervention Versus Observation Trial (PIVOT) have reported a decrease in death from all causes in men with localised prostate cancer (PCa) who underwent radical prostatectomy in the 1990s and 2000s compared to men assigned to watchful waiting [1, 2].
Results from trials conducted 20–30 years ago may not be applicable in a contemporary setting. Men diagnosed today have more favourable cancer characteristics because of earlier detection [3, 4], changes in the interpretation of the histopathological assessment of the biopsy specimen [5, 6], and an increased number of prostate biopsy cores [7] leading to stage and grade migration [8, 9] with a subsequent better survival [10–13]. This was seen in the ProtecT trial where there was no difference in PCa-specific survival after 15 years of follow-up between men diagnosed with localised PCa in 1999–2009 randomised to radical prostatectomy, radiotherapy or active monitoring [14, 15].
The aim of this study was to assess temporal trends in survival for men diagnosed with favourable-risk localised PCa managed with primary radical prostatectomy or observation. We conducted a propensity matched observational study to investigate if the survival benefit of primary radical prostatectomy remained constant over a 16-year period.
Material and methods
The National Prostate Cancer Register (NPCR) contains information of 98% of all men diagnosed with PCa in Sweden on cancer characteristics and primary treatment with the aim to audit adherence to national guidelines for PCa [16–18]. In Prostate Cancer Data Base Sweden (PCBaSe) 4.0, NPCR has been combined with data on comorbidity by use of the Charlson Comorbidity Index (CCI) based on discharge diagnoses in the Patient Registry, data on educational level and marital status from the longitudinal integrated database on socioeconomic factors (LISA), and data on cause and date of death from the Cause of Death Registry [19–24].
This study includes men aged 75 or younger with CCI 0–1 who were diagnosed with favourable-risk clinically localised PCa (T1–T2 N0/x M0/x, prostate specific antigen [PSA] <20 ng/mL and Gleason score 6 or 7 [3+4]) in the period 2000–2016, and underwent radical prostatectomy within 6 months from date of diagnosis or were managed with observation (i.e., no active treatment within 6 months from diagnosis). We stratified patients into risk groups: low-risk (T1–2 and PSA <10 ng/mL and Gleason score ≤6) and intermediate-risk (T1–2 and/or PSA 10–20 ng/mL and/or Gleason score 7 [3+4]). The following variables were used: age at diagnosis, year of diagnosis, clinical T stage, Gleason score on biopsy, PSA (ng/mL), CCI, education level, and marital status.
Follow-up was calculated from date of PCa diagnosis to death, emigration, or end of study period (31 December 2019), whichever came first. Death was classified as PCa death or non-PCa death.
The Research Ethics Board in Uppsala approved the study.
Statistical methods
Propensity score matching was performed with the MatchIt package for R using a caliper of 0.05 and included the following variables: age (<60, 60–64, 65–69, 70+), T stage (1, 2), Gleason score (6, 7 [3+4]), PSA (continuous), year of diagnosis (continuous), CCI (0, 1), education level (low, middle, high, missing), and marital status (married, not married). Follow-up was calculated with the reverse Kaplan-Meier method. Subsequent curative treatment for men primarily managed on observation was calculated as 1 – the Kaplan-Meier estimates. Cumulative incidences of PCa and non-PCa deaths with 95% CI were estimated with competing risk analyses treating deaths from other causes as competing events. Multivariable Cox regression analyses were applied to investigate the association between risk of PCa death by treatment (radical prostatectomy vs. observation), age (<60 years, 60–<65 years, 65–<70 years, 70+ years), T stage (1, 2), Gleason score (≤6, 7 (3+4)), maximum length of cancer in biopsy cores in mm (<2, 2–5, 6–12, 12+, missing), PSA (0–<5, 5–10, 10–<20), PSA density (<0.1, 01–0.15, 0.15–0.22, 0.22+, missing), CCI (0, 1), year of diagnosis, education level (low, middle, high, missing), and marital status (married, not married). The proportional hazard assumption of the Cox regression analyses was tested with Schoenfeld residuals. Full models were not applicable in all years because of few events and there were very few events in the most recent time period (2011–2016), thus the Cox analyses in this period must be interpreted with caution. The absolute risk difference was calculated as the 7-year risk of PCa death for men on observation minus the 7-year risk of PCa death for men who underwent radical prostatectomy. All tests were two-sided and the significance level was set to p < 0.05. Statistical analysis was performed with R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study included 21 149 men managed with observation (i.e., no primary curative treatment) and 18 765 men who underwent primary radical prostatectomy. The median follow-up years was 8 – 15 years for men diagnosed in 2000–2005, 10 years for men diagnosed in 2006–2010, and 5 years for men diagnosed in 2011–2016. Baseline characteristics for the whole population are presented in Table 1. More men managed with primary curative intent had adverse factors such as clinical stage T2, Gleason score 7 (3+4), or more cancer in biopsy cores, but had less comorbidities – that is more men had CCI 0. Following propensity score matching, 9 666 men were selected in each treatment strategy – 6172 with low-risk and 3494 with intermediate-risk PCa. Supplementary Tables 1 and 2 show baseline characteristics for all men and following propensity score matching stratified on risk category and diagnostic period. For both men with low- and intermediate-risk PCa there was an increase in the proportion of men diagnosed with T1 tumours in more recent calendar periods as well as a fall in the median PSA level.
The proportion of men with low-risk PCa who primarily underwent radical prostatectomy decreased for all age groups during the study period (Figure 1); whereas the proportion of men with intermediate-risk PCa who underwent radical prostatectomy remained fairly stable for men younger than 70 years and increased over time for men older than 70 years.
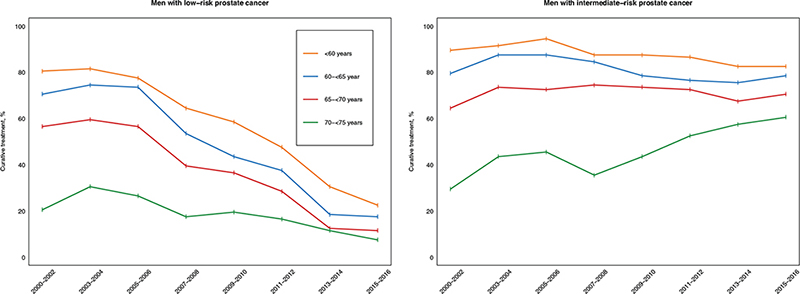
Figure 1. Proportion of men included in the study with favourable-risk localised prostate cancer who underwent primary curative treatment for each period and stratified on risk category.
The 5-year estimate for a transition to curative treatment for men with low-risk PCa managed with observation was quite stable during the study period: 26% (95% CI 24%–29%) for men diagnosed in 2000–2005, 31% (95% CI 29%–32%) for men diagnosed in 2006–2010, and 26% (95% CI 25%–27%) for men diagnosed in 2011–2016 (Figure 2). In contrast, the proportion of men with intermediate-risk PCa who transitioned to curative treatment increased, 24% (95% CI 22%–26%) in 2000–2005, 30% (95% CI 28%–32%) in 2006–2010, and 34% (95% CI 32%–35%) in 2011–2016. Of the men on observation who subsequently underwent curative treatment, 68% underwent radical prostatectomy and 32% received radiotherapy.
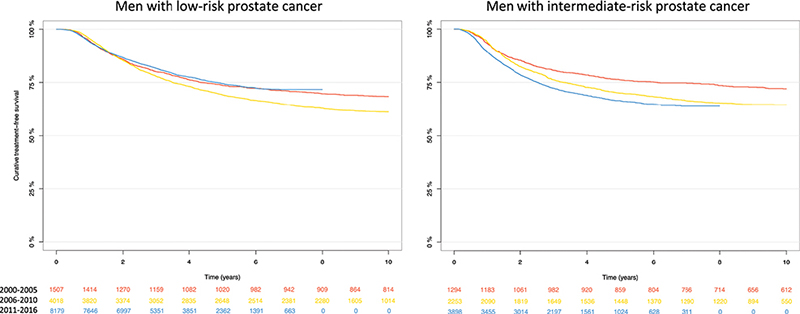
Figure 2. Kaplan-Meier estimated curative treatment-free survival for men who were managed with primary observation stratified on risk category.
The cumulative probability of PCa death stratified on treatment, time period and risk category are depicted in Figure 3. The median age at death was 77 ( interquartile range [IQR] 72–81) years for men diagnosed with low-risk PCa in 2000–2005, 73 (IQR 69–77) years for men diagnosed in 2006–2010, and 70 (IQR 66–74) years for men diagnosed in 2011–2016. Corresponding numbers for men with intermediate-risk PCa were 77 (72–81) years in 2000–2005, 74 (70–78) years 2006–2010 and 71 (68–74) years in 2011–2016. The 7-year cumulative incidence of PCa death and non-PCa death decreased by calendar period of diagnosis for both men managed with primary curative intent and men managed on primary observation (Table 2). As an example, the 7-year PCa mortality for all men with low-risk PCa managed on observation was 1.3% (95% CI 0.8–1.8) for men diagnosed in 2000–2005, 0.6% (95% CI 0.4–0.8) for men diagnosed in 2006–2010, and 0.3% (95% CI 0.01–0.4) for men diagnosed in 2011–2016. Corresponding numbers for men with intermediate-risk PCa managed on observation were 3.2% (95% CI 2.1%–4.3%) in 2000–2005, 1.6% (95% CI 0.9%–2.2%) in 2006–2010, and 0.7% (95% CI 0.3%–1.2%) in 2011–2016. Although not eliminated, the differences in non-PCa death between the treatment groups were smaller following propensity score matching compared to the raw data. As an example, the 7-year cumulative non-PCa deaths in men diagnosed with low-risk PCa in 2000–2005 was 9.8% (95% CI 8.4–11.1) for men managed on observation and 3.4% (95% CI 2.8–4.1) for men who underwent curative treatment. Corresponding numbers following propensity score matching was 8.2% (95% CI 6.7–9.7) and 4.1% (95% CI 3.0–5.2). respectively. We therefore chose only to use the propensity score matched data for further analyses.
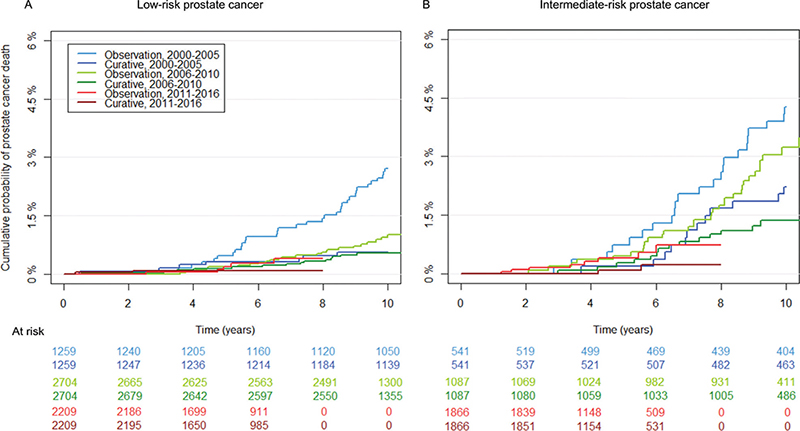
Figure 3. Cumulative probability of prostate cancer death for men with localised prostate cancer managed on primary curative treatment or observation stratified on risk category (low-risk: A, and favourable intermediate-risk B) following propensity score matching.
The risk of PCa death was lower in men with low-risk PCa managed with radical prostatectomy in 2000–2005 compared to men managed on observation, hazard ratio (HR) 0.20 (95% CI 0.10–0.38), p < 0.001 (Table 3). For men diagnosed in 2006–2010, the difference was slightly smaller, HR 0.58 (95% CI 0.29–1.14), p = 0.11, and in 2011–2016 HR was 0.35 (95% CI 0.05–2.26), p = 0.35. For men with intermediate-risk PCa, the risk was HR 0.28 (95% CI 0.16–0.47), p < 0.001, in 2000–2005, HR 0.50 (95% CI 0.26–0.95), p = 0.03, in 2006–2010, and HR 0.21 (95% CI 0.04–1.18), p = 0.08, in 2011–2016.
The absolute risk difference in risk of PCa death for men with low-risk PCa who underwent radical prostatectomy versus observation was 1.0% in 2000–2005, 0.2% in 2006–2010, and 0.4% in 2011–2016. For men with intermediate-risk PCa the absolute risk reduction was 1.1% in 2000–2005, 0.6% in 2005–2010, and 0.7% in 2011–2016.
Discussion
In this propensity score matched analysis of men with favourable-risk localised PCa, those men managed by primary radical prostatectomy had a lower relative risk of PCa death compared to men managed with observation – but the absolute difference decreased in more recent calendar time. The cumulative risk of PCa death at 7 years in men managed with primary observation declined in both men with low- and favourable intermediate-risk PCa. These changes are likely a consequence of stage and grade migration caused by earlier detection, and changes in clinical work-up and histopathological assessment.
A general limitation of observational studies is the risk of selection bias. Although propensity score matching was used to make the groups more similar, the difference in non-PCa death indicates that factors not controlled for influenced the outcome. Additionally, the propensity score matching did not eliminate differences in cancer extent in the biopsies. We did not have information on some important information such as subgroup cT2 stage neither type of observational strategy (watchful waiting or active surveillance) nor adherence to proper follow-up for men on active surveillance or the cause for transit to curative treatment. With approximately 1/4 men changing from observation to curative treatment after 5 years, these data are in line with previously published active surveillance cohort studies [25]. Additionally, the median follow-up was only 5 years for men diagnosed in 2011–2016; therefore, we can only assess the short-term outcome for a disease for which longer follow-up is necessary. The number of men with intermediate-risk PCa managed with observation increased three-fold between 2000–2005 and 2011–2016. Thus, wider inclusion criteria of observation were applied and this likely resulted in a larger proportion of men with progression and transition to curative treatment. Additionally, there is a risk of bias in the cause of death registration [26]. The overall agreement on the cause of death registered in the Swedish Death Registry and following patient chart review has been reported to be high [27, 28]. In more recent studies from Estonia, Norway, and Sweden, the concordance between adjudicated cause of death in the Cause of Death Register and assessment of charts have been lower [28–30]. In Sweden there was a high likelihood for older men with low-risk PCa to have death adjudicated to PCa despite little evidence of disease progression [28]. Speculatively, incorrect cause of death will be more frequent in men on observation, which would overestimate the benefit of surgery compared to initial observation.
Our study investigated if the survival benefit of radical prostatectomy compared to observation, has changed over time in a clinical practise in order to aid the interpretation of randomised studies comparing surgery to observation in men with PCa during different calendar periods. Importantly, the current study did not compare outcome after radical prostatectomy and active surveillance. First, we must keep in mind that ‘initial observation’ has changed during the last decades. 20–30 years ago, ‘observation’ meant watchful waiting where men who progressed received androgen deprivation therapy. During the last 20 years, active surveillance has been introduced to reduce overtreatment with ensuing adverse effects of treatment in men with low and favourable intermediate-risk PCa without compromising PCa-specific survival. Active surveillance aims to identify men who progress and to offer them curative treatment when the disease is still within the window of curability. Several randomised trials have compared outcome after radical prostatectomy and watchful waiting or active monitoring [1, 14, 31, 32]. The SPCG-4 and PIVOT both reported a decrease in death from all causes in men with localised PCa who underwent radical prostatectomy compared to men assigned to watchful waiting [1, 2]. The absolute risk reduction of PCa death in men randomised to radical prostatectomy was 11% compared to watchful waiting after 23 years of follow-up in the SPCG-4 trial that recruited men between 1989 and 1999 [33], and 4% after 19 years in the more recent PIVOT trial with recruitment in 1994–2002 [32]. Corresponding reductions in the absolute risk of death from all causes were 13% and 6%, respectively. There were large differences in case mix in these studies. SPCG-4, a Scandinavian trial included non-screened men of whom 88% had T2 tumours and the mean PSA was 13 ng/mL. Men in the North American PIVOT trial mostly had screen-detected PCa, of whom 45% had T2 tumours and the median PSA was 8 ng/mL. In the more recent ProtecT study comparing surgery, radiation, and active monitoring, men were diagnosed following PSA testing in 1999–2009, where 76% had T1c tumours and the median PSA was 4.8 ng/mL, reported no difference in all cause or PCa-specific survival at 15 years [14]. In line with these randomised studies, the absolute survival benefit of radical prostatectomy compared to observation declined in more recent calendar periods in our study. Men treated with radical prostatectomy had a lower risk of PCa death compared to men on observation; however, survival for men with favourable-risk PCa has improved over time regardless of treatment. We question if an absolute difference of 0.5% in PCa mortality after 7 years of follow-up is clinically relevant. Of note, we could not differentiate between watchful waiting and active surveillance, so our results are likely a worst-case scenario.
Stage and grade migration have occurred for PCa [8, 9], which has resulted in better prognosis of men with PCa [10–13]. An increase uptake of PSA testing in asymptomatic combined with an increase in the number of biopsy cores [13], has led to an increased detection [34], of non-palpable PCa [3, 4]. Furthermore, changes in histopathological assessment of prostate biopsies have [5, 6], resulted in a grade inflation [35, 36].
Conclusion
The temporal decrease in risk of PCa death both for men treated with radical prostatectomy and observation is likely caused by stage and grade migration. The decrease in difference in absolute survival between these two groups in more recent calendar periods suggests that men currently diagnosed with low-risk and favourable intermediate-risk PCa have a smaller benefit from primary curative treatment compared to men in historical studies.
Author contributions
All authors contributed to the manuscript and approved of the final version. FT had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. There were no other contributors to the manuscript except those on the author list.
Acknowledgement
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Elin Axén, Johan Styrke, Johan Stranne, Jon Kindblom, Camilla Thellenberg, Andreas Josefsson, Ingrida Verbiene, Hampus Nugin, Stefan Carlsson, Anna Kristiansen, Mats Andén, Thomas Jiborn, Olof Ståhl, Olof Akre, Per Fransson, Eva Johansson, Magnus Törnblom, Fredrik Jäderling, Marie Hjälm Eriksson, Lotta Renström, Jonas Hugosson, Ola Bratt, Maria Nyberg, Fredrik Sandin, Camilla Byström, Mia Brus, Mats Lambe, Anna Hedström, Nina Hageman, Christofer Lagerros, and patient representatives Hans Joelsson, and Gert Malmberg.
Data availability statement
Data used in the present study were extracted from the Prostate Cancer Data Base Sweden (PCBaSe), which is based on the National Prostate Cancer Register (NPCR) of Sweden. The data cannot be shared publicly because the individual-level data contain potentially identifying and sensitive patient information and cannot be published due to legislation and ethical approval (https://etikprovningsmyndigheten.se). Use of the data from national health data registers is further restricted by the Swedish Board of Health and Welfare (https://www.socialstyrelsen.se/en/) and Statistics Sweden (https://www.scb.se/en/) which are Government Agencies providing access to the linked healthcare registers.
References
- [1] Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up. N Engl J Med. 2018;379:2319–2329. https://doi.org/10.1056/NEJMoa1807801
- [2] Wilt TJ, Vo TN, Langsetmo L, et al. Radical prostatectomy or observation for clinically localized prostate cancer: extended follow-up of the Prostate Cancer Intervention Versus Observation Trial (PIVOT). Eur Urol. 2020;77(3):713–724. https://doi.org/10.1016/j.eururo.2020.02.009
- [3] Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;6736:1–9. https://doi.org/10.1016/S0140-6736(14)60525-0
- [4] Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. https://doi.org/10.1016/S1470-2045(10)70146-7
- [5] Epstein JI, Allsbrook WC, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. https://doi.org/10.1097/01.pas.0000173646.99337.b1
- [6] Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. https://doi.org/10.1097/PAS.0000000000000530
- [7] Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175(5):1605–1612. https://doi.org/10.1016/S0022-5347(05)00957-2
- [8] Moore AL, Dimitropoulou P, Lane A, et al. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int. 2009;104:1592–1598. https://doi.org/10.1111/j.1464-410X.2009.08652.x
- [9] Danneman D, Drevin L, Robinson D, Stattin P, Egevad L. Gleason inflation 1998–2011: a registry study of 97,168 men. BJU Int. 2015;115:248–255. https://doi.org/10.1111/bju.12671
- [10] Berg KD, Thomsen FB, Nerstrøm C, et al. The impact of the 2005 International Society of Urological Pathology consensus guidelines on Gleason grading – a matched pair analysis. BJU Int. 2016;117:883–889. https://doi.org/10.1111/bju.13439
- [11] Cazzaniga W, Garmo H, Robinson D, et al. Mortality after radical prostatectomy in a matched contemporary cohort in Sweden compared to the Scandinavian Prostate Cancer Group 4 (SPCG-4) study. BJU Int. 2019;123:421–428. https://doi.org/10.1111/bju.14563
- [12] Thomsen FB, Folkvaljon Y, Brasso K, et al. Prognostic implications of 2005 Gleason grade modification. Population-based study of biochemical recurrence following radical prostatectomy. J Surg Oncol. 2016;114(6):664–670. https://doi.org/10.1002/jso.24408
- [13] Thomsen FB, Garmo H, Brasso K, Egevad L, Stattin P. Temporal changes in cause-specific death in men with localised prostate cancer treated with radical prostatectomy. A Population-based, nationwide study. J Surg Oncol. 2021;124(5):867–875. https://doi.org/10.1002/jso.26579
- [14] Hamdy FC, Donovan JL, Lane JA, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388:1547–1558. https://doi.org/10.1056/NEJMoa2214122
- [15] Thomsen FB, Jakobsen H, Langkilde NC, et al. Active surveillance for localized prostate cancer: nationwide observational study. J Urol. 2019;201(3):520–527. https://doi.org/10.1016/j.juro.2018.09.045
- [16] Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 1: early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand J Urol. 2022;56:265–273. https://doi.org/10.1080/21681805.2022.2094462
- [17] Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 2: recurrent, metastatic and castration resistant disease. Scand J Urol. 2022;56:278–284. https://doi.org/10.1080/21681805.2022.2093396
- [18] Stattin P. How to survey adherence to guidelines by use of clinical cancer registers. Scand J Urol. 2022;56:285–286. https://doi.org/10.1080/21681805.2022.2107069
- [19] Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42:956–967. https://doi.org/10.1093/ije/dys068
- [20] Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015;54:158–163. https://doi.org/10.3109/0284186X.2014.939299
- [21] Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51:101–111. https://doi.org/10.1016/j.ejca.2014.10.025
- [22] Berglund A, Garmo H, Tishelman C, et al. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol. 2011;185:833–839. https://doi.org/10.1016/j.juro.2010.10.061
- [23] Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34:423–437. https://doi.org./10.1007/s10654-019-00511-8
- [24] Godtman R, Holmberg E, Stranne J, Hugosson J. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45:226–232. https://doi.org/10.3109/00365599.2011.559950
- [25] Thomsen FB, Brasso K, Klotz LH, et al. Active surveillance for clinically localized prostate cancer – a systematic review. J Surg Oncol. 2014;109:830–835. https://doi.org/10.1002/jso.23584
- [26] Bergengren O, Westerberg M. Watch out for sticky diagnosis bias in older men with prostate cancer. Scand J Urol. 2022;56:365–366. https://doi.org/10.1080/21681805.2022.2124305
- [27] Fall K, Stromberg F, Rosell J, et al. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–357. https://doi.org/10.1080/00365590802078583
- [28] Orrason AW, Styrke J, Garmo H, et al. Evidence of cancer progression as the cause of death in men with prostate cancer in Sweden. BJU Int. 2023;131:486–493. https://doi.org/10.1111/bju.15891
- [29] Innos K, Paapsi K, Alas I, et al. Evidence of overestimating prostate cancer mortality in Estonia: a population-based study. Scand J Urol. 2022;56:359–364. https://doi.org/10.1080/21681805.2022.2119274
- [30] Löffeler S, Halland A, Weedon-Fekjær H, et al. High Norwegian prostate cancer mortality: evidence of over-reporting. Scand J Urol. 2018;52:122–128. https://doi.org/10.1080/21681805.2017.1421260
- [31] Iversen P, Madsen P, Corle D. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate. Twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl. 1995;172:65–72.
- [32] Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132–142. https://doi.org/10.1056/NEJMoa1615869
- [33] Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. https://doi.org/10.1056/NEJMoa1311593
- [34] Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. https://doi.org/10.1016/j.eururo.2012.02.054
- [35] Dong F, Wang C, Farris AB, et al. Impact on the clinical outcome of prostate cancer by the 2005 international society of urological pathology modified Gleason grading system. Am J Surg Pathol. 2012;36:838–843. https://doi.org/10.1097/PAS.0b013e3182486faf
- [36] Grogan J, Gupta R, Mahon KL, et al. Predictive value of the 2014 International Society of Urological Pathology grading system for prostate cancer in patients undergoing radical prostatectomy with long-term follow-up. BJU Int. 2017;120:651–658. https://doi.org/10.1111/bju.13857