ORIGINAL RESEARCH ARTICLE
Triggers for transition from active surveillance to radical treatment of prostate cancer 2008–2020 – a case-control study
Mats S. Ahlberga , Hans Garmoa,b
, Hans Garmoa,b , Pär Stattina
, Pär Stattina , Rolf Gedeborga
, Rolf Gedeborga , Christer Edlundc, Lars Holmberga,b
, Christer Edlundc, Lars Holmberga,b and Anna Bill-Axelsona
and Anna Bill-Axelsona
aDepartment of Surgical Sciences, Uppsala University, Uppsala, Sweden; bTranslational Oncology & Urology Research (TOUR), School of Cancer and Pharmaceutical Sciences, King’s College London, UK; cUrologkliniken, Hallands sjukhus, Kungsbacka, Sweden
ABSTRACT
Objective: To examine associations between objective signs of progression (triggers) and transition from active surveillance (AS) to radical treatment for prostate cancer (PC).
Patients and methods: This case-control study included men with low- or favourable intermediate-risk PC in the region of Halland, with data from The National Prostate Cancer Register (NPCR), Sweden, starting AS between 2008 and 2020. Cases were men who transitioned to radical treatment. For each case, 10 controls who remained in AS were selected without further matching. Triggers for transition to treatment were histopathological progression, magnetic resonance imaging (MRI) progression and increases in prostate-specific antigen (PSA) levels. We compared the probabilities for triggers between cases and controls, in 2008–2014 and 2015–2020, using logistic regression.
Results: Amongst 846 men, we identified 98 cases in 2008–2014 and 172 cases in 2015–2020. Histopathological progression was associated with transition, most strongly in the later period (2008–2014: odds ratios [OR] 6.88, 95% confidence interval [CI] 3.69–12.80; and 2015–2020: OR 75.29, 95% CI 39.60–143.17). MRI progression was associated with transition in 2015–2020 (OR 6.38, 95% CI 2.70–15.06), whereas an increase in PSA was weakly associated with transition in the early period. The absence of triggers was associated with no transition (2008–2014: OR 0.24, 95% CI 0.15–0.40, and 2015–2020: OR 0.09, 95% CI 0.06–0.14). The probability of no trigger was 27% in cases 2015–2020.
Conclusion: The increase in association between histopathological trigger and transition to treatment indicates increased quality of AS. Still, amongst men treated from 2015 to 2020, 27% transitioned without any trigger.
KEYWORDS: Prostate cancer; active surveillance; triggers for transition to radical treatment
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 63–69. https://doi.org/10.2340/sju.v59.34803.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 8 December 2023; Accepted: 10 February 2024; Published: 14 March 2024
CONTACT Mats S. Ahlberg mats.ahlberg@uu.se Department of Surgical Sciences, Akademiska sjukhuset, Ing 70, SE-751 85 Uppsala, Sweden
Competing interests and funding: The authors report no conflicts of interest apart from Rolf Gedeborg who is employed by the Medical Products Agency (MPA) in Sweden. The MPA is a Swedish Government Agency. The views expressed in this article may not represent the views of the MPA.
This study was supported by Swedish Cancer Society, the Swedish Prostate Cancer Society, the Percy Falk Foundation, the Foundation of the memory of Johanna Hagstrand and Sigfrid Linnér and the Hillevi Fries Foundation. The funders of the study had no role in conduct, data collection, management, analysis and interpretation of the study, preparation or approval of the manuscript, or the decision to submit for publication.
Introduction
Transition from active surveillance (AS) to radical treatment for prostate cancer (PC) should ideally be triggered by objective signs of progression. Histopathological progression in repeated biopsy is a key trigger [1]. However, biopsies are invasive and uncomfortable and have potential side effects such as bleeding and infection, and adherence to repeated re-biopsies is poor [2–4]. Magnetic Resonance Imaging (MRI) is recommended for men in AS, yet the evidence for its importance for long-term outcomes of AS is weak [1, 5–8]. Refraining from intervention is often safe in the absence of MRI progression if prostate-specific antigen (PSA) levels are stable, but specific criteria for MRI changes indicative of disease progression during AS remain elusive [9]. PSA doubling time and PSA velocity have been used as indicators of disease progression. Currently, PSA density is increasingly replacing PSA only [10, 11]. PSA levels are affected by many factors other than PC, and PSA increase is not recommended as a sole trigger for treatment [1, 11]. Whilst digital rectal examination is established in diagnosing locally advanced disease, its sensitivity for progression in organ confined tumours remains unproven [1]. Treatment initiation based on patient or physician preference, without a specific trigger, is common but leads to overtreatment [12]. The way triggers are used in clinical practice and how this has changed over time are unknown [5, 8].
We analysed the probabilities of experiencing triggers – histopathological progression, MRI progression and PSA increase – for transitioning from AS to radical treatment for PC, and their association with transition to radical treatment. We used a register-based case-control study to investigate how this association changed with the introduction of prostate MRI under the hypothesis of increasingly strong associations between triggers and treatment over time, indicating stronger adherence to guidelines recommending objective indications for transition to treatment.
Method
The National Prostate Cancer Register (NPCR) of Sweden captures 98% of all PC cases in Sweden compared with the Swedish Cancer Registry, where all diagnosed cancers are registered by law, and collects data about tumour characteristics at diagnosis [13, 14]. Prostate Cancer data Base Sweden (PCBaSe) is a research database in which NPCR has been linked with the National Patient Register, the Swedish Cancer Register, the Cause of Death Register, the Prescribed Drug Register, the Multi-Generation Register and the Longitudinal integrated database for health insurance and labour market studies (LISA), using the unique Swedish personal identity number [15, 16]. Additional healthcare data from one healthcare region (Region Halland) were cross-linked with NPCR at the National Board of Health and Welfare in April 2023. Data on PSA, prostate MRIs and prostate biopsies after PC diagnosis were extracted [14].
Identification of cases and controls
Men in Region Halland, who started AS between 1 January 2008 and 30 June 2020, remaining in AS for at least 6 months and who met criteria similar to those in the Prostate Cancer Active Surveillance Trigger trial/Scandinavian Prostate Cancer Group study no. 17 – PCASTt/SPCG17 (Clinical T-stage 1 (cT1) – cT2, PSA < 15 ng/mL, PSA density ≤ 0.2 ng/mL2, any amount of Gleason score 6 or Gleason score 3 + 4 = 7 in less than 30% of cores and <10 mm cancer in any core containing Gleason score 3 + 4 = 7) with a life expectancy of at least 10 years, were identified [17]. All men meeting the above criteria and who transitioned from AS to radical treatment were selected as cases. For each case, 10 controls remaining in AS 3 months after the time of radical treatment for their corresponding case were randomly selected without further matching. We pre-defined two separate time periods based on the date of radical treatment: one early period from 2008 to 2014, before introduction of prostate MRI in Swedish guidelines, and one late period from 2015 to 2020, after its introduction in guidelines.
Exposure
We defined three mutually exclusive triggers for transitioning from AS to radical treatment.
- Histopathological trigger, with or without any other progression, defined as increase in Gleason score from ≤6 to Gleason score ≥3 + 4 = 7, or from Gleason score 3 + 4 = 7 to ≥4 + 3 = 7.
- MRI trigger, with or without PSA progression but without histopathological progression, according to PCASTt/SPCG17 criteria (≥5 mm progression in largest tumour-diameter or progression in highest reported Prostate Imaging Reporting and Data System [PIRADS] score on repeated prostate MRI) [17]. Prostate MRI reports were evaluated by one author (MSA), blinded to the outcomes. The highest PIRADS score and longest diameter of visible lesion were obtained for MRI progression assessment. MRI was present as a trigger only in the late period.
- PSA trigger, without other progression, defined as PSA doubling time less than 3 years, PSA increase ≥2 ng/mL or PSA density increase of ≥0.05 ng/mL2 over a 2-year period. PSA progression was evaluated using linear regression considering the three preceding years for PSA doubling time and the two preceding years for PSA velocity and PSA density.
Exposures included triggers reached within 1 year before date of radical treatment.
Assessment of life expectancy
For life expectancy estimation, we used an improvement of the previously described statistical model based on Charlson Comorbidity Index (CCI), Drug Comorbidity Index (DCI) and age [18–20]. In the improved model, a multi-dimensional diagnosis-based index (MDCI) replaces CCI, which improves its predictive performance [21]. Life expectancy dropping below 10 years was considered transition to watchful waiting.
Statistical methods
Continuous data are presented as median and interquartile ranges (IQR) or mean with 95% confidence interval (CI), as appropriate. Categorical data are presented as proportions and percentages.
Associations between potential triggers and transition from AS to radical treatment were estimated using conditional logistic regression and presented as odds ratios (OR) with 95% CI. We used three models: an unadjusted model and two multivariable models. The first adjusted for patient and tumour characteristics, and the second also included socioeconomic factors. Baseline covariates relevant to the outcome, all listed in Table 1, were incorporated in the multivariable model to assess their additional impact on the associations. Redundant covariates ‘risk group’ and ‘mm cancer in biopsies’ were excluded from the adjusted analysis. We also conducted a sensitivity analysis including triggers reached within 2 and 3 years before the date of treatment. To visualise the continuous change in probability of triggers, we used an unadjusted logistic regression model, with natural cubic splines, to capture non-linear relationships in the data. The model included three knots at 27.5th, 50th and 72.5th percentiles, and two boundary knots at 5th and 95th percentiles along the predictor variable [22].
This study was approved by the Research Ethics Authority and adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for case-control studies [23].
Results
The study base included 846 men with 1,073 biopsy-rounds, 687 prostate MRIs and 8,947 PSA tests (Table 1). We identified 98 cases in the early period and 172 cases in the late period. AS was allowed to start in 2008 for both periods, and thus, the median time in AS was longer in the late period. Tumour characteristics, except for PSA, showed slightly more advanced features in cases compared with controls. Approximately 90% were Gleason score 6, cT1 and classified as low risk PC. The majority had CCI 0.
Probability of experiencing triggers
In the early period, the probability of a histopathological trigger was 30% for cases and 5% for controls (Table 2). For PSA trigger, the probability was 34% for cases and 19% for controls, whilst the probability of not experiencing any trigger was 37% for cases and 75% for controls.
In the late period, the probability of a histopathological trigger was 48% for cases and 2% for controls, and for MRI trigger, 6% for cases and 1% for controls. The probability of PSA trigger was 19% for both cases and controls, whilst the probability of not experiencing any trigger was 27% for cases and 79% for controls. In a subgroup analysis of men having experienced both a histopathological trigger the year before transition to treatment, and an MRI trigger within 3 years before transition, the probability was 7.6% (Table 2). The same analysis for the period 2017–2020, excluding the years before MRI was introduced in the Swedish guidelines, showed a probability of 9.2%.
In the unadjusted continuous analysis of trigger probabilities, there was a decreasing probability for cases and an increasing probability for controls of not having experienced any trigger the year before the date of treatment (Figure 1). Furthermore, in the unadjusted continuous visualisation, most of the increase in probability of histopathological trigger in cases occurred before 2016, and MRI trigger emerged in 2017. The probability of experiencing PSA-triggers before treatment varied substantially throughout the whole study period (Figure S1).
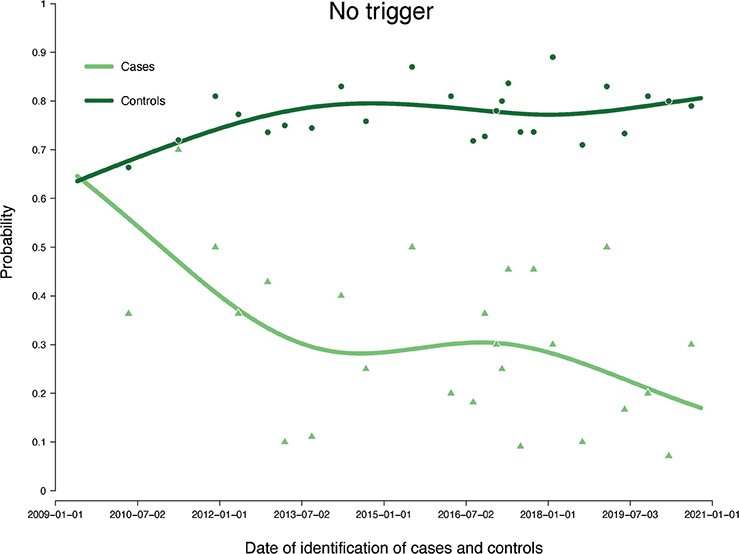
Figure 1. Probabilities for cases and controls of not having experienced any trigger the year before date of treatment. Illustrated by an unadjusted logistic regression model and with point estimates of means of 11 consecutive observations. Natural cubic splines were used to smoothen the non-linear relationship and fit it to a linear curve, illustrating the probabilities continuously over the entire study period.
Association between triggers and radical treatment
In the early period, histopathological trigger was associated with transition to radical treatment in both the unadjusted logistic regression model (OR 7.66, 95% CI 4.49–13.06) and the adjusted models (OR 7.54, 95% CI 4.15–13.70 and OR 6.88, 95% CI 3.69–12.80) (Table 2). For PSA trigger, the association was weaker. Not having experienced any trigger was associated with not transitioning to radical treatment (unadjusted model: OR 0.19, 95% CI 0.12–0.29, adjusted models: OR 0.23, 95% CI 0.14–0.36 and OR 0.24, 95% CI 0.15–0.40).
In the late period, histopathological trigger was associated with transition to radical treatment in the unadjusted (OR 53.01, 95% CI 30.02–93.62) and adjusted models (OR 67.28, 95% CI 36.16–125.18 and OR 75.29, 95% CI 39.60–143.17). MRI trigger was associated with treatment (unadjusted model: OR 6.32, 95% CI 2.82–14.17, adjusted models: OR 5.85, 95% CI 2.52–13.57 and OR 6.38, 95% CI 2.70–15.06), whilst PSA trigger was not. Not having experienced any trigger was associated with not transitioning to radical treatment (unadjusted model: OR 0.10, 95% CI 0.07–0.15, adjusted models: OR 0.09, 95% CI 0.06–0.14 and OR 0.06, 95% CI 0.06–0.14) (Table 2). The sensitivity analysis did not change the main results (Table S1).
Discussion
The probability of a histopathological trigger amongst men who transitioned to treatment increased over time. An association between MRI trigger and transition to treatment emerged following the introduction of MRI in Swedish guidelines. The probability of a PSA trigger for men who transitioned to treatment was lower in the later period, and the probability of no trigger before treatment decreased during the study period.
Neither PSA progression nor MRI progression is recommended as triggers to radical treatment if not accompanied by histopathological progression [1]. In a previous study in NPCR with data from 2008 to 2013, 24% of the men transitioned from AS to radical treatment within 5 years due to biopsy progression [24]. In the PRIAS and GAP3 studies, 34% and 28%, respectively, transitioned from AS to radical treatment within 5 years, due to a mixture of reasons suggesting disease progression [12, 25]. Direct comparison with the current study is difficult due to different study designs and definitions of disease progression, but in all studies, histopathological progression often precedes transition from AS to radical treatment, in accordance with guidelines [1]. Our study showed a strong association between histopathological progression and transition to radical treatment, particularly in the later period. The probability of a histopathological trigger increased over time in cases, reaching nearly 50% in the late period, suggesting better adherence to guidelines over time.
Data from the PRIAS study demonstrated that PIRADS ≥ 3 on prostate MRI was a statistically significant predictor of histopathological progression on targeted biopsies during AS [9]. PRIAS data also showed that MRI before starting AS reduces the probability of discontinuing AS within two years, and an increasing probability of discontinuing AS when MRI was performed during AS [26]. The favourable outcomes of AS without MRI, along with the increasing probability of upgrading and discontinuation of AS with the usage of MRI and targeted biopsies, raise concerns for potential overtreatment when integrating prostate MRI into AS without well-defined guidelines [27, 28]. This could possibly be balanced by increased use of MRI contributing to better adherence to guidelines and reduced risk of discontinuation the first 2 years, which could also contribute to the longer time in AS seen in the later period of this study. A systematic review focusing on prostate MRI’s reliability in detecting PC progression during AS found moderate accuracy in identifying histopathological upgrading and poor accuracy in ruling out histopathological upgrading [29]. This implies that MRI alone may not be a sufficient criterion for excluding true disease progression and may not be accurate enough to indicate histopathological progression.
Since 2014, prostate MRI has been recommended by the Swedish PC guidelines. In the study population, the number of MRIs increased from occasional examinations before 2011 to around one hundred in 2016 and kept increasing every year thereafter (data not shown) with 55 MRIs registered in the early period and 632 in the late period. Radiology novices show low segmentation accuracy and high interindividual variation, which might be applicable to the early period in the current study [30]. In the late period, an association between MRI progression and transition to radical treatment emerged, but the probability of having experienced an MRI trigger before treatment was still low. In our subset analysis, there was a probability of only 8% for treated men having experienced both a histopathological trigger within 1 year before treatment and an MRI trigger within 3 years preceding treatment, suggesting that the introduction of prostate MRI played little role in the increased probability of histopathological trigger before treatment during this period.
In the previous study from NPCR, 52% of men who transitioned to radical treatment did so due to PSA progression [24]. In the PRIAS 5-year follow-up, 46% of men who transitioned to radical prostatectomy after PSA doubling time of less than 3 years had favourable prostatectomy-specimen histopathology, which underscores the limited predictive value of PSA progression for histopathological progression [11, 12]. The probability of PSA increase only as a trigger in cases decreased between the two time periods. There was a weak association with treatment in the early period, but in the late period, the estimates for an association with transition to treatment were close to unity. This is possibly suggesting an increasing adherence to guidelines, and PSA increase alone should not normally trigger transition. It is supported by the sensitivity analysis, where there were tendencies of lower ORs in the late period compared with the early period.
In men who transition to radical treatment, previous studies have shown that between 13% and 30% of men do so without evidence of disease progression [24, 25] Approximately 60% of men who transition without any known disease progression exhibit favourable histopathology in prostatectomy specimens, indicating overtreatment [12]. Avoiding overtreatment is important to avoid common side effects of radical treatment, for example urinary incontinence after radical prostatectomy [31] The ORs of not having experienced any trigger were low in our study and decreased between the study periods, demonstrating a robust association between no trigger and no transition to radical treatment. The consistent decline in no trigger before transition can possibly be attributed to increased awareness amongst healthcare professionals about the safety of AS. However, the probability of no trigger the year before treatment was still 27% in cases in the late period, indicating that subjective preference by the patient and/or doctor remained one of the major reasons for transition to treatment.
In the continuous analysis, the increasing probability of histopathological trigger in cases, before MRI was widely used, does not support the hypothesis that the introduction of MRI directly influenced the increased use of that trigger. The emergence of MRI trigger in 2017 suggests that reliance on MRI progression being indicative of true disease progression started a few years after the introduction of prostate-MRI. However, the model is unadjusted and can, therefore, be biased, and the variation in PSA trigger was likely due to few events and by variability in utilisation of PSA trigger.
Strengths and limitations
To our knowledge, this is the first registry-based study assessing objective triggers for transition from AS to radical treatment for PC. Strengths of our study include that completeness and validity is high in NPCR and the administrative healthcare registers [13]. Limitations of this study include that there were some prostate biopsies, MRIs and PSA tests, performed outside the Halland region and by one private healthcare facility, missing in the database, introducing information bias. The relatively small cohort size from one healthcare region is another limitation. However, the Halland region consists of urban and rural communities, and according to NPCR data, the use of AS for low- and intermediate-risk PC was in line with the rest of Sweden [14]. The results may, therefore, be generalisable to a broader context with similar PC occurrence and healthcare organisation.
Conclusion
The increase in the association between triggers, particularly histopathological trigger, and transition to radical treatment indicates improved adherence to guidelines over time. Prostate MRI contributed only modestly as a trigger. Despite a decline in the probability of no trigger before transition, still more than a quarter of men transitioned without a trigger, and more than half transitioned without histopathological progression. The lack of objective triggers before transition indicates a need for further improvement to reduce overtreatment during AS.
Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden steering group: Elin Axén, Johan Styrke, Andreas Josefsson, Camilla Thellenberg, Ingrida Verbiené, Hampus Nugin, Stefan Carlsson, Anna Kristiansen, Mats Andén, Kimia Kohestani, Jon Kindblom, Thomas Jiborn, Olof Ståhl, Olof Akre, Eva Johansson, Magnus Törnblom, Fredrik Jäderling, Marie Hjälm Eriksson, Lotta Renström Koskela, Jonas Hugosson, Ola Bratt, Erik Thimansson, Johan Stranne, Elin Trägårdh, Viktoria Gaspar, Fredrik Sandin, Petrus Stenson, Lena Pettersson, Mia Brus, Gustaf Hedström, Anna Hedström, Maria Moutran, Nina Hageman, Maria Nyberg, Hans Joelsson and Gert Malmberg.
ORCID
Mats S. Ahlberg https://orcid.org/0000-0002-2404-5890
Hans Garmo https://orcid.org/0000-0001-7181-7083
Pär Stattin https://orcid.org/0000-0002-8306-0687
Rolf Gedeborg https://orcid.org/0000-0002-8850-7863
Lars Holmberg https://orcid.org/0000-0003-4417-7396
Anna Bill-Axelson https://orcid.org/0000-0002-4559-1217
References
- [1] Lam TBL, MacLennan S, Willemse PPM, et al. EAU-EANM-ESTRO-ESUR-SIOG prostate cancer guideline panel consensus statements for Deferred Treatment with Curative Intent for Localised Prostate Cancer from an International Collaborative Study (DETECTIVE Study). Eur Urol. 2019;76(6):790–813.
- [2] Olsson H, Nordström T, Clements M, et al. Intensity of active surveillance and transition to treatment in men with low-risk prostate cancer. Eur Urol Oncol. 2020;3(5):640–647. https://doi.org/10.1016/j.euo.2019.05.005
- [3] Bokhorst LP, Alberts AR, Rannikko A, et al. Compliance rates with the prostate cancer research international active surveillance (PRIAS) protocol and disease reclassification in noncompliers. Eur Urol. 2015;68(5):814–821. https://doi.org/10.1016/j.eururo.2015.06.012
- [4] Lundström KJ, Drevin L, Carlsson S, et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014;192(4):1116–1122. https://doi.org/10.1016/j.juro.2014.04.098
- [5] EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023_2023-06-13-141145.pdf [Internet]. [cited 23-09-2023]. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023_2023-06-13-141145.pdf
- [6] www.nccn.org.pdf [Internet]. [cited 23-09-2023]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- [7] Guidelines – American Urological Association [Internet]. [cited 24-11-2021]. Available from: https://www.auanet.org/guidelines-x15197
- [8] Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 1: early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand J Urol. 2022;56(4):265–273. https://doi.org/10.1080/21681805.2022.2094462
- [9] Luiting HB, Remmers S, Boevé ER, et al. A multivariable approach using magnetic resonance imaging to avoid a protocol-based prostate biopsy in men on active surveillance for prostate cancer—data from the international multicenter prospective PRIAS study. Eur Urol Oncol. 2022;5(6):651–658. https://doi.org/10.1016/j.euo.2022.03.007
- [10] Omri N, Kamil M, Alexander K, et al. Association between PSA density and pathologically significant prostate cancer: The impact of prostate volume. Prostate. 2020;80(16):1444–1449. https://doi.org/10.1002/pros.24078
- [11] Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol [Internet]. 2016 [cited 23-04-2023]. Available from: https://ascopubs.org/doi/pdf/10.1200/JCO.2008.18.1685?role=tab
- [12] Bokhorst LP, Valdagni R, Rannikko A, et al. A decade of active surveillance in the PRIAS study: An update and evaluation of the criteria used to recommend a Switch to active treatment. Eur Urol. 2016;70(6):954–960. https://doi.org/10.1016/j.eururo.2016.06.007
- [13] Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol Stockh Swed. 2015;54(2):158–163. https://doi.org/10.3109/0284186X.2014.939299
- [14] NPCR [Internet]. [cited 02-12-2022]. Available from: https://statistik.incanet.se/npcr/
- [15] Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42(4):956–967. https://doi.org/10.1093/ije/dys068
- [16] Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. Int J Epidemiol. 2016;45(1):73–82. https://doi.org/10.1093/ije/dyv305
- [17] Ahlberg MS, Adami HO, Beckmann K, et al. PCASTt/SPCG-17-a randomised trial of active surveillance in prostate cancer: rationale and design. BMJ Open. 2019;9(8):e027860. https://doi.org/10.1136/bmjopen-2018-027860
- [18] Van Hemelrijck M, Ventimiglia E, Robinson D, et al. Population-based estimates of age and comorbidity specific life expectancy: a first application in Swedish males. BMC Med Inform Decis Mak. 2022;22(1):35. https://doi.org/10.1186/s12911-022-01766-0
- [19] Gedeborg R, Sund M, Lambe M, et al. An aggregated comorbidity measure based on history of filled drug prescriptions: development and evaluation in two separate cohorts. Epidemiol Camb Mass. 2021;32(4):607–615. https://doi.org/10.1097/EDE.0000000000001358
- [20] Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
- [21] Westerberg M, Irenaeus S, Garmo H, Stattin P, Gedeborg R. Development and validation of a multi-dimensional diagnosis-based comorbidity index that improves prediction of death in men with prostate cancer: Nationwide, population-based register study. PLoS One. 2024;19(1):e0296804. https://doi.org/10.1371/journal.pone.0296804
- [22] Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020;55(4):675–680. https://doi.org/10.1038/s41409-019-0679-x
- [23] STROBE [Internet]. [cited 08-10-2023]. Checklists. Available from: https://www.strobe-statement.org/checklists/
- [24] Loeb S, Folkvaljon Y, Makarov DV, et al. Five-year nationwide follow-up study of active surveillance for prostate cancer. Eur Urol. 2015;67(2):233–238. https://doi.org/10.1016/j.eururo.2014.06.010
- [25] Van Hemelrijck M, Ji X, Helleman J, et al. Reasons for discontinuing active surveillance: assessment of 21 centres in 12 countries in the Movember GAP3 consortium. Eur Urol. 2019;75(3):523–531.
- [26] Luiting HB, Remmers S, Valdagni R, et al. What is the effect of MRI with targeted biopsies on the rate of patients discontinuing active surveillance? A reflection of the use of MRI in the PRIAS study. Prostate Cancer Prostatic Dis. 2021;24(4):1048–1054. https://doi.org/10.1038/s41391-021-00343-2
- [27] Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(3):272–277. https://doi.org/10.1200/JCO.2014.55.1192
- [28] Hamdy FC, Donovan JL, Lane JA, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547–1558. https://doi.org/10.1016/j.eururo.2023.08.014
- [29] Rajwa P, Pradere B, Quhal F, et al. Reliability of serial prostate magnetic resonance imaging to detect prostate cancer progression during active surveillance: a systematic review and meta-analysis. Eur Urol. 2021;80(5):549–563. https://doi.org/10.1016/j.eururo.2021.05.001
- [30] Langkilde F, Masaba P, Edenbrandt L, et al. Manual prostate MRI segmentation by readers with different experience: a study of the learning progress. Eur Radiol. 2024. https://doi.org/10.1007/s00330-023-10515-4
- [31] Arnsrud Godtman R, Persson E, Bergengren O, et al. Surgeon volume and patient-reported urinary incontinence after radical prostatectomy. Population-based register study in Sweden. Scand J Urol. 2022;56(5–6):343–350. https://doi.org/10.1080/21681805.2022.2119270