ORIGINAL RESEARCH ARTICLE
Anorectal function and symptoms 6 months after robot-assisted laparoscopic radical prostatectomy: a single-center study
Theodoros Psariasa, Susanna Walterb, Martin Holmboma, Issa Khayoun Issaa, Firas Abdul-Sattar Aljaberya and Olof Hallböökc
aDepartment of Clinical and Experimental Medicine, Division of Urology, Linköping University, Linköping, Sweden; bDivision of Diagnostics and Specialist Medicine, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden; cDepartment of Biomedical and Clinical Sciences, Division of Surgery, Linköping University, Linköping, Sweden
ABSTRACT
Introduction: Robot-assisted laparoscopic radical prostatectomy (RALP) is a common procedure for the treatment of localised prostate cancer. Anorectal symptoms such as fecal incontinence (FI), rectal urgency or disturbed defecation have been reported after the operation. Anorectal function is dependent on the integrity of anal and pelvic nerves and muscles, rectal sensory function as well as rectal reservoir function.
The aim of this study was to investigate the potential influence of RALP on anorectal physiological function and bowel symptoms.
Materials and Methods: In this pilot study, 29 patients with localised prostate cancer scheduled for RALP were included. Anorectal physiology was used to measure rectal sensitivity and reservoir function as well as anal sphincter pressures. Bowel symptoms were measured by a bowel function questionnaire and a 2-week bowel function diary. Measurements were done before the operation and repeated at 6 months after the operation.
Results: The study observed a significant postoperative increase in rectal sensory threshold for rectal balloon distention, from 20 to 40 mmHg, P < 0.001. This change is indicative of a decrease in rectal sensation after RALP. There were no other statistical significant differences in any of the physiological tests performed. Importantly, there was no change in any of the bowel symptoms after surgery.
Conclusion: This study showed that RALP may lead to impaired rectal sensory function. This finding did not, however, seem to have any influence on the patients´ postoperative clinical bowel function.
KEYWORDS: RALP; prostatectomy; fecal incontinence; bowel; anorectal function
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 104–108. https://doi.org/10.2340/sju.v59.35396.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 28 December 2023; Accepted: 22 March 2024; Published: 13 May 2024
CONTACT Theodoros Psarias theodoros.psarias@regionostergotland.se Department of Clinical and Experimental Medicine, Division of Urology, Linköping University, Linköping, Sweden
Competing interests and funding: The authors report no conflict of interest.
Introduction
The incidence of prostate cancer worldwide is currently increasing. In Sweden, prostate cancer is the most common cancer in men, with about 10,000 new cases each year [1].
Radical prostatectomy (RP) is a standard treatment for localised disease. The first reported robot-assisted surgery was in 2000, and since then robot-assisted laparoscopic radical prostatectomy (RALP) has become accepted worldwide [2, 3]. The indication of RP is cancer control, but postoperative both short-term and long-term complications such as urinary and sexual dysfunction remain of concern [4–6]. Furthermore, post-RALP patients sometimes present symptoms such as FI, rectal urgency, and disturbed defecation [7].
Anorectal physiological function is complex and dependent on intricate neuropathway mechanisms and the integrity of anal and pelvic nerves, muscles and reflexes, rectal sensory function as well as rectal reservoir function.
RALP, being performed close to the rectum, has the potential to influence the sensory innervation of the rectum and the pelvic floor muscles. The parasympathetic neural innervation to the colon plays a significant role in regulating propulsive colonic motility prior defecation. Damage to these nerves, particularly the sacral root branches from S1 to S5, can cause a sympathetic upregulation of the distal bowel. Consequently, RALP could potentially change distal bowel function causing clinical manifestations such as changes in bowel habits or control of bowel contents.
Recent epidemiological studies indicate that FI occurs in up to 15% of the western population and impairs the quality of life [8].
In a large population-based survey, the prevalence of FI was 8% and 5.4% by Rome criteria. It was more prevalent among women 9.1% than men 7.4% [9]. A comparison was made between men and women with FI regarding their symptoms and pathophysiological mechanisms. The study revealed that in men, impaired rectal sensation and functional disturbances of evacuation were more prominent, while anal sphincter dysfunction was less common compared to women [10].
We hypothesised that RALP, due to the relevant surgical anatomy, could negatively affect the innervation to the distal bowel and cause changes in bowel habits or control of bowel contents.
The aim of the study was to investigate anorectal function and symptoms in patients with prostate cancer following a RALP intervention.
Materials and methods
Study design and population
In this single-center pilot study, a cohort of 29 patients was examined before and after the surgery, resulting in two groups for comparison, the patients acting as their own controls.
Patients diagnosed with localised prostate cancer and scheduled for RALP at Linkoping University Hospital were recruited for this study between 2011 and 2013. The patients received verbal and printed information about the study and provided a written informed consent before participating. The study protocol was approved by the Swedish National Ethic Committee (Dnr 2010-07007). Exclusion criteria were known bowel disease, previous radiation therapy in the abdominal area, previous anorectal surgery, or unfit for the study for demographical, psychological, or social reasons.
A total of 30 men diagnosed with localised prostate cancer were included. One patient refrained from the postoperative measurements, resulting in a final sample size of 29 participants (Table 1).
The bowel function questionnaire and bowel diary were administered to the patient upon inclusion in the study [11]. The patients were instructed to record their bowel habits in the diary on a daily basis for 14 days. They recorded details of every stool, including consistency (loose, normal, or hard), frequency of bowel motion, occurrence of feces or gas leakage, urgency prior to defecation, as well as a detailed documentation of use of pads or diapers.
One to two weeks before the operation the patients were called for anorectal physiology testing at the Pelvic Floor Unit, Linkoping University Hospital. The same research nurse performed the anorectal measurements in all patients.
At 6 months after RALP, the patients were asked to repeat the bowel function questionnaire and bowel diary. The anorectal physiological testing was also repeated.
Anorectal manometry
A microtransducer system was used to assess the anorectal pressure profile. The catheter with a diameter of 1.7 mm was introduced into the rectum, and the resting and straining pressures were recorded at defined distances from the anal verge using manual station pull-through technique. Sphincter function was assessed based on the maximum resting and straining pressures and the area under the resting and straining pressure curves. The resting and straining pressure area under the curve (AUC) was measured and calculated at a distance of 0 to 5 cm from the anal verge to include the high-pressure zone in the anal canal [12].
Rectal manovolumetry
A mechanical barometric system, as described by Hallböök & Sjödahl was used to measure rectal volume changes during isobaric distensions [13]. With the patient in the left lateral position, a non-compliant polyethylene bag attached to a polyethylene tube was inserted into the rectum. The tube was connected to the barostat and held in position by the investigator. The rectal volumes at each distention pressure increment and sensitivity thresholds were recorded (ascending method of limits). Between the distensions the balloon was emptied completely. The subjects were instructed to notify three sensation thresholds: (1) first sensation of filling, (2) first sensation of urge and (3) maximum tolerable distention.
Surgical technique
The RALP technique was identical for all the patients and performed by two surgeons using the da Vinci S surgical system. With a transperitoneal approach, the prostate was dissected close to the bladder neck. No lymph node dissection or nerve-sparing was carried out, and all operations were deemed radical. The vas deferens and seminal vesicles were dissected free and pulled upward to reveal the Denonvilliers’ fascia under tension. Using sharp and blunt dissection, dissection behind the prostate began in the plane between the rectum and prostate by separation of the prostate and Denonvilliers fascia from the rectum. The distal limit of this dissection was the apex of the prostate. Urethrovesical anastomosis was then performed.
Statistical methods
All results were analysed using the GraphPad Prism 9 software. The physiological results were not normally distributed, therefore non-parametric tests were used. The bowel questionnaire produced ordinal data. A P-value < 0.05 was considered statistically significant. Data were presented as medians and interquartile ranges (IQR) unless otherwise specified. Paired data were analysed by the Wilcoxon signed-rank test. Missing data due to loss of follow-up or physiological testing malfunction were managed through pairwise deletion.
Results
Anorectal physiology measurements
We observed a statistically significant postoperative increase in the pressure needed to elicit a sensation of filling the rectum, from a median of 20 mmHg before the operation to 40 mmHg postoperatively (Table 2 and Figure 1). This difference was statistically significant (P < 0.001). Resting and straining pressure variables, including the pressures in the area under the curve (AUC), showed no significant differences pre- versus post-operatively, see Figure 1. The various parameters regarding rectal sensitivity and manovolumetry is shown in Table 2. Rectal volumes at the different sensation thresholds also remained unchanged.
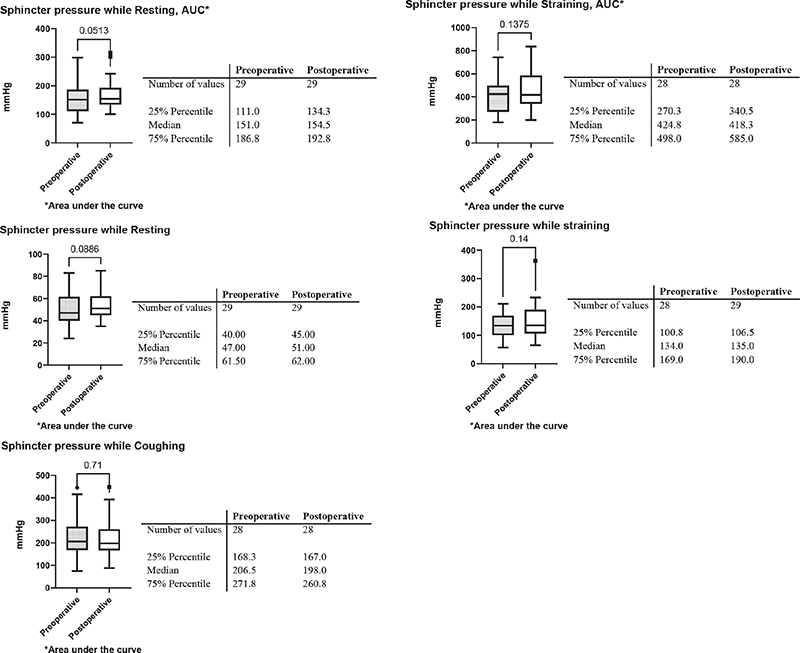
Figure 1. Anorectal pressure parameters. N = 29. The Wilcoxon singed rank test was used for group comparisons. Sphincter pressure while resting, straining and coughing pre- and post-operative.
Bowel function questionnaire and diary
After the operation, no significant differences were found regarding bowel symptoms reported by the patients in the questionnaire, see Table 3. Similarly, no statistically significant difference was found regarding number of stools per week or stool consistency. Fecal and gas leakage episodes also remained unchanged after the operation, see Table 4.
Discussion
Over the last decade, RALP has emerged as the dominant surgical method for radical prostatectomy. In addition to cancer-related outcomes, there is a need to investigate the postoperative quality of life of patients undergoing RALP.
Fecal incontinence, characterized by the inability to control the anal sphincter resulting in accidental passage of stool or gas, can be of concern in patients with prostate cancer receiving treatment. Specifically, Fusco et al. reported FI in 5% and 18% of patients undergoing radical retropubic prostatectomy and radical perineal prostatectomy, respectively.
In this study, we evaluated anorectal physiological functions and distal bowel symptoms in patients with prostate cancer planned for RALP and repeated the evaluation in the same patients 6 months after the operation.
To achieve this, we employed a barostat setup, which allowed for sensitivity and volume/pressure measurements in the rectum. In addition to that, anal pressure measurements during resting and straining were performed to provide an assessment of anal sphincter function.
The only statistically significant change after the operation was an increase of the distension pressure in the rectum needed to elicit a sensation of filling. This finding indicates a postoperative decrease in rectal sensitivity. There were no other statistically significant differences seen in any of the physiological tests performed.
Even though this was an isolated finding, it raises the possibility that RALP could have a negative impact on rectal sensitivity. A possible explanation for this finding could be an operative injury affecting the innervation of the rectum. However, it is worth noting that this finding of decreased rectal sensitivity does not seem to be reflected in the results obtained from the clinical bowel function tests, as assessed by the bowel function questionnaire and bowel diary. In theory, rectal hyposensitivity is associated with disturbed defecation or fecal incontinence [14]. However, in our material we found no statistically significant clinical impact, a finding that is in line with previous, similar studies [7, 15]. This result is relevant to clinical practice and specifically to the challenging selection of treatment for localised prostate cancer. The alternative to radical surgery is radiotherapy, which, however, seems to a greater extent has a negative impact on bowel function [16, 17].
Interestingly, we also observed a small numerical increase in postoperative sphincter pressures, although this change was not statistically significant. A possible explanation for this could be the indirect effect of pelvic floor muscle exercises that the patients performed after surgery, indirectly resulting in a small improvement of their anal sphincter function.
To our knowledge, there is currently no specific method to assess anorectal symptoms after RALP or urological surgery in general. As a result, we used a previously published, although not validated bowel function questionnaire as well as validated bowel diary [18]. These methods are widely accepted in the field of gastroenterology and colorectal surgery, ensuring a high level of internal validity and reliability in addressing the primary aim of our study [7, 12].
The main strength of the study and the main difference from previous publications is the comprehensive set-up of anorectal physiology testing and bowel symptoms assessment. In addition, the patients acted as their own controls and thus, paired statistical analyses were used. The validated bowel diary was designed as a prospective diagnostic instrument to be used in studies that examine various patient groups with a broad spectrum of GI symptoms [18, 19].
There are certain limitations that should be acknowledged. The small sample size limits the generalizability of the findings. In addition, the small sample size limited the possibility to further divide the sample into smaller groups according to different nerve sparing grades of the operation. Another limitation is the relatively short time to postoperative follow up, only 6 months after surgery. The questionnaire has not been validated, nor were the psychometric properties tested. As the questionnaire was primarily constructed in the Swedish language, any translational issues are not relevant. Lastly, there is a possibility that the observed decrease in rectal sensitivity may resolve after a longer postoperative period [15].
Importantly, this only physiologic finding was not reflected in any of the bowel symptoms assessed at 6 months after surgery either by the questionnaire or the bowel diary.
In this pilot study we found no support for that robot-asssisted laparoscopic prostatectomy deteriorates bowel function in a clinical meaningful way.
Acknowledgments
The authors would like to acknowledge Marie Rosberg, research assistant nurse, Division of Diagnostics and Specialist Medicine, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden.
References
- [1] Regionala cancercentrum i samverkan. Nationellt vårdprogram för prostatacancer; version 8.1. [Internet]. Stockholm: Regionala cancercentrum i samverkan; 2023. [citerad 2023-12-08]. https://kunskapsbanken.cancercentrum.se/diagnoser/prostatacancer/.
- [2] Rassweiler J, Frede T, Seemann O, et al. Telesurgical laparoscopic radical prostatectomy. Initial experience. Eur Urol. 2001;40(1):75–83. https://doi.org/10.1159/000049752
- [3] Laviana AA, Williams SB, King ED, et al. Robot assisted radical prostatectomy: the new standard? Minerva Urol Nefrol. 2015;67(1):47–53.
- [4] Tutolo M, Bruyneel L, Van der Aa F, et al. A novel tool to predict functional outcomes after robot-assisted radical prostatectomy and the value of additional surgery for incontinence. BJU Int. 2021;127(5):575–584. https://doi.org/10.1111/bju.15242
- [5] Geraghty K, Keane K, Davis N. Systematic review on urinary continence rates after robot-assisted laparoscopic radical prostatectomy. Ir J Med Sci. 2024. https://doi.org/10.1007/s11845-023-03603-3
- [6] Kennady EH, Zillioux J, Ali M, et al. Longitudinal urgency outcomes following robotic-assisted laparoscopic prostatectomy. World J Urol. 2023;41(7):1885–1889. https://doi.org/10.1007/s00345-023-04458-0
- [7] Aydemir H, Albayrak S, Canguven O, et al. Anorectal functions after perineal and retropubic radical prostatectomy – a prospective clinical and anal manometric assessment. Arch Med Sci. 2011;7(1):138–142. https://doi.org/10.5114/aoms.2011.20619
- [8] Walter S, Hjortswang H, Holmgren K, et al. Association between bowel symptoms, symptom severity, and quality of life in Swedish patients with fecal incontinence. Scand J Gastroenterol. 2011;46(1):6–12. https://doi.org/10.3109/00365521.2010.513059
- [9] Mack I, Hahn H, Gödel C, Enck P, Bharucha AE. Global prevalence of fecal incontinence in community-dwelling adults: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023.
- [10] Townsend DC, Carrington EV, Grossi U, et al. Pathophysiology of fecal incontinence differs between men and women: a case-matched study in 200 patients. Neurogastroenterol Motil. 2016;28(10):1580–1588. https://doi.org/10.1111/nmo.12858
- [11] Hallböök O, Påhlman L, Krog M, et al. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg. 1996;224(1):58–65. https://doi.org/10.1097/00000658-199607000-00009
- [12] Sjodahl J, Walter SA, Johansson E, et al. Combination therapy with biofeedback, loperamide, and stool-bulking agents is effective for the treatment of fecal incontinence in women – a randomized controlled trial. Scand J Gastroenterol. 2015;50(8):965–974. https://doi.org/10.3109/00365521.2014.999252
- [13] Hallböök OJ, Sjödahl RI. Compliance and manovolumetry. In: Wexner SD, Duthie GS, editors. Constipation. London: Springer; 2006. p. 99–103.
- [14] Karlbom U, Lundin E, Graf W, et al. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis. 2004;6(5):343–349. https://doi.org/10.1111/j.1463-1318.2004.00632.x
- [15] Nishikawa R, Honda M, Teraoka S, et al. Effects of nerve-sparing procedures on bowel function after robot-assisted radical prostatectomy: a longitudinal study. Int J Med Robot. 2020;16(6):1–10. https://doi.org/10.1002/rcs.2156
- [16] Corsini C, Bergengren O, Carlsson S, et al. Patient-reported side effects 1 year after radical prostatectomy or radiotherapy for prostate cancer: a register-based nationwide study. Eur Urol Oncol. 2024 Jan 16. https://doi.org/10.1016/j.euo.2023.12.007
- [17] Wang Z, Ni Y, Chen J, et al. The efficacy and safety of radical prostatectomy and radiotherapy in high-risk prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2020;18(1):42. https://doi.org/10.1186/s12957-020-01824-9
- [18] Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10(5):415–421. https://doi.org/10.1097/00042737-199805000-00011
- [19] Ragnarsson G, Bodemar G. Division of the irritable bowel syndrome into subgroups on the basis of daily recorded symptoms in two outpatients samples. Scand J Gastroenterol. 1999;34(10):993–1000. https://doi.org/10.1080/003655299750025093