ORIGINAL RESEARCH ARTICLE
Risk of prostate cancer death in men diagnosed with prostate cancer at cystoprostatectomy. A nationwide population-based study
Pietro Scilipotia,b , Fredrik Liedbergc,d
, Fredrik Liedbergc,d , Hans Garmoa
, Hans Garmoa , Andri Wilberg Orrasona
, Andri Wilberg Orrasona , Pär Stattina
, Pär Stattina and Marcus Westerberga
and Marcus Westerberga
aDepartment of Surgical Sciences, Uppsala University, Uppsala, Sweden; bDivision of Experimental Oncology/Unit of Urology, IRCCS Ospedale San Raffaele, Milan, Italy; cDepartment of Urology Skåne University Hospital, Malmö, Sweden; dInstitution of Translational Medicine, Lund University, Malmö, Sweden
ABSTRACT
Background and aims: One out of three men who undergo cystoprostatectomy for bladder cancer is diagnosed with incidental prostate cancer (PCa) at histopathological examination. Many of these men are PSA tested as part of their follow-up, but it is unclear if this is needed. The aim of this study was to assess the risk of PCa death in these men and the need of PSA-testing during follow-up.
Methods: Between 2002 and 2020, 1,554 men were diagnosed with PCa after cystoprostatectomy performed for non-metastatic bladder cancer and registered in the National Prostate Cancer Register (NPCR) of Sweden. We assessed their risk of death from PCa, bladder cancer and other causes up to 15 years after diagnosis by use of data in The Cause of Death Register. The use of androgen deprivation therapy (ADT) as a proxy for PCa progression was assessed by fillings in The Prescribed Drug Register.
Results: Fifteen years after diagnosis, cumulative incidence of death from PCa was 2.6% (95% CI 2.3%–2.9%), from bladder cancer 32% (95% CI: 30%–34%) and from other causes 40% (95% CI: 36%–44%). Only 35% of men with PCa recorded as primary cause of death in The Cause of Death Register had started ADT before date of death, indicating sticky-diagnosis bias with inflated risk of PCa death.
Conclusions: For a large majority of men diagnosed with incidental PCa at cystoprostatectomy performed for bladder cancer, the risk of PCa death is very small so there is no rationale for PSA testing during follow-up.
KEYWORDS: Bladder cancer; cystoprostatectomy; incidental prostate cancer; risk of death
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 98–103. https://doi.org/10.2340/sju.v59.40001.
Copyright: © 2024 The Author(s). Published by Medical Journals Sweden on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 30 January 2024; Accepted: 02 April 2024; Published: 13 May 2024
CONTACT Marcus Westerberg marcus.westerberg@uu.se Regional Cancer Center Midsweden, Uppsala University Hospital, SE-752 37, Uppsala, Sweden
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v59.40001
Competing interests and funding: The authors report no conflicts of interest.
This project was supported by The Swedish Research Council (2022-00544), The Swedish Cancer Society [19 00 30], Region Uppsala and Uppsala University. The sponsors had no involvement with the planning, execution, or completion of the study.
Introduction
Incidental prostate cancer (PCa) is diagnosed in one out of three men who undergo cystoprostatectomy for bladder cancer [1–3]. Several studies have described that a majority of these cancers are ‘clinically insignificant’ at histopathological examination of the surgical specimen [2, 3], whereas other studies have cautioned about the aggressiveness of these cancers [4, 5]. Many men diagnosed with PCa at cystoprostatectomy for bladder cancer are PSA tested during follow-up; however, the benefit of such testing is questionable [6–8].
The aim of our study was to assess the long-term risk of PCa death in a large representative cohort of men diagnosed with PCa at cystoprostatectomy performed for bladder cancer in order to inform on the need of PSA testing during follow-up.
Methods
The primary aim of the National Prostate Cancer Register (NPCR) of Sweden, a clinical cancer register, is to ensure high quality of care for men with PCa and adherence to national guidelines [9, 10]. In Prostate Cancer data Base Sweden (PCBaSe) version 5.0, data for men registered in NPCR up to 2020-12-30 have been linked to other nationwide, population-based Swedish healthcare registers and demographic databases, including The Patient Register, The Cancer Register, The Prescribed Drug Register, The Longitudinal integrated database for health insurance and labour market studies (LISA), a socioeconomic database, The Emigration Register and The Cause of Death Register, as a basis for clinical research [11].
Men diagnosed with PCa between 2002 and 2020 registered in the NPCR were included if they had a non-metastatic bladder cancer diagnosis according to The Cancer Register and had undergone a cystoprostatectomy according to The In-Patient Register with a PCa diagnosed up to 7 days prior to or 60 days after the date of surgery (Supplementary Table S1). The time window for the PCa diagnosis in relation to the date of the cystoprostatectomy was set in consideration of the expected time required for obtaining the pathological report. We conducted sensitivity analyses by exploring different time windows for inclusion.
We extracted data on PCa including Gleason Score, postoperative Tumor, Node, Metastases (TNM) stage from the NPCR and on bladder cancer including urinary diversion and postoperative TNM stage from The In-Patient Register and The Cancer Register (Supplementary Table S1). Educational level [low (mandatory school), middle (high school) and high (university)] and income (defined in quartiles) were extracted from The LISA register. The use of androgen deprivation therapy (ADT; gonadotropin releasing hormone, antiandrogens, or surgical castration) was identified by use of data on filled prescriptions in The Prescribed Drug Register (available from July 1, 2005) and date of castration in The In-Patient Register. The Charlson Comorbidity Index (CCI) was calculated based on diagnostic codes extracted from The In-Patient Register, excluding PCa and bladder cancer [12]. Date and cause of death (prostate, bladder and other) were extracted from The Cause of Death Register.
Follow-up started at the date of PCa diagnosis. Men were followed until date of migration, date of death, or until 2022-12-31, whichever event came first.
We assessed the competing risks of death using cumulative incidence curves stratified for Gleason 6, Gleason 7 and Gleason ≥ 8 and age < 75 and ≥ 75 years at diagnosis. For all statistical analyses, R version 4.3.2 was used [13].
The Swedish Research Ethics Authority approved the study.
Results
A total of 184,916 men diagnosed with PCa were registered in NPCR between 2002 and 2020. Of these men, 1,554 (1%) men underwent radical cystoprostatectomy due to non-metastatic bladder cancer up to 7 days before the date of PCa diagnosis or up to 60 days after surgery. Around 300 men per year undergo cystoprostatectomy for bladder cancer in Sweden, corresponding to around 5,400 men between 2002 and 2020 [14]. According to these estimates, 29% (1,554/5,400) men in Sweden who underwent cystoprostatectomy for bladder cancer were diagnosed with incidental PCa.
Out of men with incidental PCa, 1,277 men (82%) were assigned a Gleason score, and amongst these, 843 (66%) had Gleason 6, 379 (30%) had Gleason 7 and 55 (4%) had Gleason ≥ 8 (Table 1). Median age was 72 years (Q1–Q3: 67–77), and 585 (38%) men were older than 75 years. Preoperative PSA was missing in 77% of the men. Median preoperative PSA was 3 ng/mL (Q1–Q3: 1–5 ng/mL) in the 360 men who had a PSA on record before the date of surgery.
| All | % | Gleason 6** | % | Gleason 7** | % | Gleason ≥ 8** | % | Missing Gleason | % | |
| N | 1,554 | 100 | 843 | 100 | 379 | 100 | 55 | 100 | 277 | 100 |
| Age at prostate cancer diagnosis, years | ||||||||||
| Median (Q1–Q3) | 72 (67–77) | 71 (65–76) | 74 (69–78) | 74 (72–80) | 73 (69–77) | |||||
| <65 | 331 | 21 | 223 | 26 | 52 | 14 | 5 | 9 | 51 | 18 |
| 65–75 | 638 | 41 | 341 | 40 | 151 | 40 | 24 | 44 | 122 | 44 |
| 75+ | 585 | 38 | 279 | 33 | 176 | 46 | 26 | 47 | 04 | 38 |
| Charlson Comorbidity Index | ||||||||||
| 0 | 924 | 60 | 499 | 59 | 226 | 60 | 32 | 58 | 167 | 60 |
| 1 | 287 | 18 | 167 | 20 | 61 | 16 | 9 | 16 | 50 | 18 |
| 2 | 177 | 11 | 98 | 12 | 49 | 13 | 8 | 15 | 22 | 8 |
| 3+ | 166 | 11 | 79 | 9 | 43 | 11 | 6 | 11 | 38 | 14 |
| Year of diagnosis | ||||||||||
| 2002–2011 | 588 | 38 | 415 | 49 | 128 | 34 | 26 | 47 | 19 | 7 |
| 2012–2015 | 429 | 28 | 183 | 22 | 99 | 26 | 13 | 24 | 134 | 48 |
| 2016–2020 | 537 | 35 | 245 | 29 | 152 | 40 | 16 | 29 | 124 | 45 |
| Preoperative PSA, ng/mL | ||||||||||
| Median (Q1–Q3) | 3 (1–5) | 2 (1–4) | 4 (2–6) | 7 (5–10) | 2 (0–4) | |||||
| <2 | 154 | 10 | 111 | 13 | 37 | 10 | 2 | 4 | 4 | 1 |
| 2–4 | 94 | 6 | 62 | 7 | 29 | 8 | 2 | 4 | 1 | 0 |
| 4+ | 112 | 7 | 47 | 6 | 49 | 13 | 14 | 25 | 2 | 1 |
| Missing | 1,194 | 77 | 623 | 74 | 264 | 70 | 37 | 67 | 270 | 97 |
| Civil status | ||||||||||
| Spouse or partner | 980 | 63 | 531 | 63 | 246 | 65 | 34 | 62 | 169 | 61 |
| No spouse or partner | 574 | 37 | 312 | 37 | 133 | 35 | 21 | 38 | 108 | 39 |
| Income | ||||||||||
| Q1 | 413 | 27 | 220 | 26 | 115 | 30 | 16 | 29 | 62 | 22 |
| Q2 | 457 | 29 | 244 | 29 | 118 | 31 | 24 | 44 | 71 | 26 |
| Q3 | 319 | 21 | 180 | 21 | 81 | 21 | 5 | 9 | 53 | 19 |
| Q4 | 237 | 15 | 126 | 15 | 41 | 11 | 4 | 7 | 66 | 24 |
| Missing | 128 | 8 | 73 | 9 | 24 | 6 | 6 | 11 | 25 | 9 |
| Level of education* | ||||||||||
| Low | 605 | 39 | 351 | 42 | 145 | 38 | 19 | 35 | 90 | 32 |
| Middle | 628 | 40 | 340 | 40 | 158 | 42 | 24 | 44 | 106 | 38 |
| High | 321 | 21 | 152 | 18 | 76 | 20 | 12 | 22 | 81 | 29 |
| *Low: less than 10 years (mandatory school), intermediate: 10–12 years (high school), high: more than 12 years of education (university). | ||||||||||
| **International Society of Urological Pathologists (ISUP) Grade Group: Gleason 6 = ISUP 1; Gleason 7 = ISUP 2–3; Gleason ≥ 8 = ISUP 4–5. | ||||||||||
Pathological stage T2 of the bladder cancer was registered for 721 (46%) of the men (Table 2). The distribution of pT stage and the pN stage was similar in men with Gleason 6, Gleason 7 and Gleason ≥ 8 (Table 2). A large majority of men (80%) received a ureteroenterocutaneostomy (ileal conduit) and (13%) received a neobladder (Supplementary Table S2).
At 15 years of follow-up, cumulative incidence of PCa death for the full study group was 2.6% (95% CI: 2.3%–2.9%), and specifically 1.2% (95% CI: 1.0%–1.4%) for men with Gleason 6, 4.3% (95% CI: 3.3%–5%) for Gleason 7 and 13% (95% CI: 9%–19%) for Gleason ≥ 8 (Figure 1). Amongst men with Gleason 6, the risk of death from bladder cancer was 34% (95% CI: 31%–38%), and death from other causes was 35% (95% CI: 31%–40%) at 15 years. In men with Gleason 7, the corresponding risks were 28% (95% CI: 25%–32%) and 52% (95% CI: 41%–66%), respectively, and for men with Gleason ≥ 8, outcomes were similar as for men with Gleason 7 (Figure 1). Men with unknown Gleason had similar risk of death from PCa and other causes as the full study group.
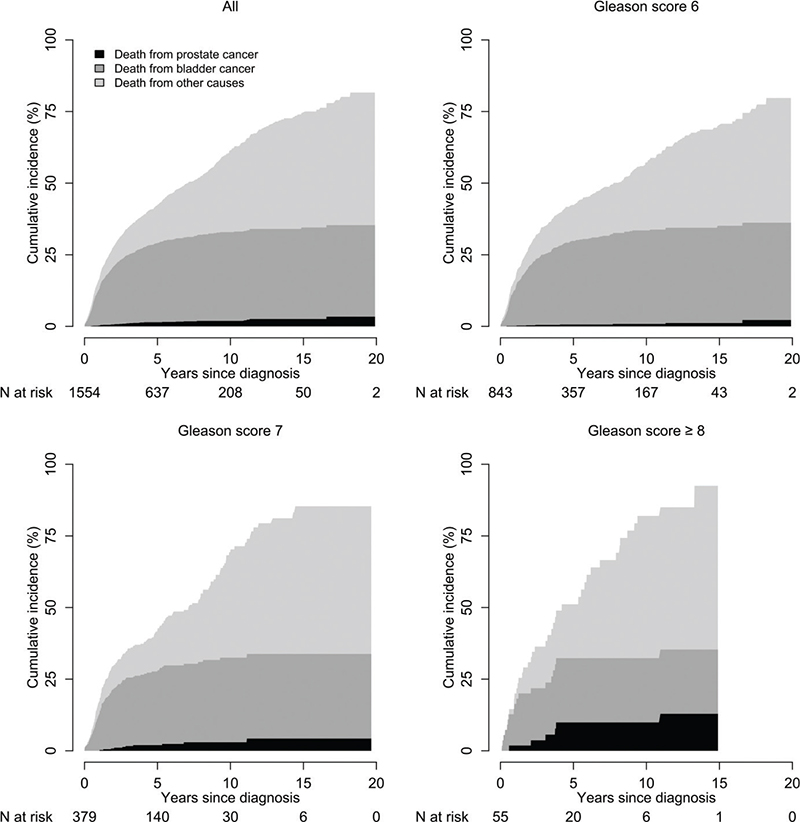
Figure 1. Cumulative incidence of death from prostate cancer, bladder cancer and other causes after cystoprostatectomy performed for non-metastatic bladder cancer in 2002–2020, stratified for Gleason score.
Cumulative incidence of PCa death at 15 years increased with age, from 1.6% (95% CI: 1.4%–1.9%) in men aged below 75 years (n = 969) to 4.2% (95% CI: 3.6%–5%) in men aged ≥75 years (n = 585). Same trend was observed for the risk of death from bladder cancer in men below 75 years compared to men aged ≥75 years, 28% (95% CI: 25%–31%) versus 39% (95% CI 35%–44%) as well as for death from other causes, 36% (95% CI: 31–41) vs 49% (95% CI: 41%–58%) (Figure 2).
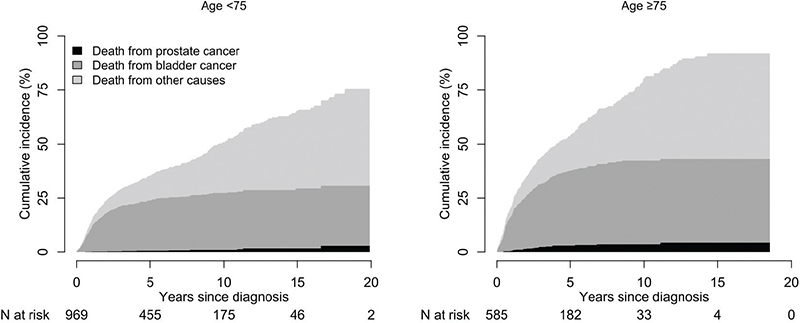
Figure 2. Cumulative incidence of death from prostate cancer, bladder cancer and other causes after cystoprostatectomy performed for non-metastatic bladder cancer in 2002–2020 stratified for age.
Out of the 29 men who had PCa recorded as the primary cause of death in The Cause of Death Register, 30% had Gleason 6 and only 40% had bladder cancer as a contributing cause of death. Amongst the 26 of these men who resided in regions where the use of ADT was recorded in The Prescribed Drug Register, only 9 (35%) men had initiated ADT before the date of death, with a higher proportion amongst men with Gleason ≥ 8 [5 out of 6 (83%)].
The findings were robust to changes in inclusion criteria in two sensitivity analyses, using alternative definitions based on 30 days prior to or 7 days after date of PCa diagnosis (n = 1,544), and 60 days prior to or 1 day after date of PCa diagnosis (n = 1,406) (data not shown).
Discussion
In this nationwide, population-based study of men diagnosed with PCa after cystoprostatectomy performed for bladder cancer, the risk of PCa death at 15 years was below 3%, risk of death from bladder cancer was 32%, and risk from death from other causes was 40% according to The Cause of Death Register.
Strengths of our study include that NPCR captures virtually all men (> 98%) diagnosed with PCa in Sweden compared to the Cancer Register, to which registration is mandated by law [10, 11]. The Cancer Register and The National Patient Register are nationwide, population-based administrative registers with documented high validity [15–19]. Furthermore, we had access to data on use of ADT in The Prescribed Drug Register during follow-up for a large majority of men who had PCa recorded as primary cause of death in The Cause of Death Register. By use of these data, we could assess the likelihood of PCa progression before the date of death. To our knowledge, this study is larger, is based on more comprehensive data and has longer follow-up than previous studies on this topic [20, 21].
Inherent limitations of our study due to the characteristics of men diagnosed with incidental PCa included that we lacked data on preoperative PSA for a majority of the men, and we also lacked pre-operative tumour stage since there was no suspicion of PCa diagnosis before surgery. Furthermore, there were no data on PSA during follow-up, since neither The NPCR nor any of the other health care registers in PCBaSe hold data on PSA. We had no data on use of cisplatin-based chemotherapy prior to cystoprostatectomy, which putatively could have decreased detection of PCa.
Incidental PCa diagnosed in men who undergo cystoprostatectomy for bladder cancer is common. Approximately, one out of three men in Sweden who undergo cystoprostatectomy for bladder cancer are diagnosed with PCa at the histopathological examination of the surgical specimen. Similar proportions of incidental PCa at cystoprostatectomy have been reported in previous studies. In a systematic review and meta-analysis of 13,140 men, 24% men were diagnosed with PCa after cystectomy [2], and other studies that have reported similar proportions [3, 4, 5, 22]. The type of pathological assessment of the prostate is likely to affect detection of PCa. In a study in which whole-mount sectioning was performed, 36% of the specimens contained PCa [22]. Extensive pathological examination has likely become more common in Sweden during the study period, and we observed a slight increase of incidental PCa diagnoses over calendar time corroborating that a more extensive pathological examination increases the detection of PCa [4]. Currently, the proportion of men with latent PCa detected at autopsy is similar, that is around 30% in many Western countries, indicating that the same pool of undetected clinically insignificant PCa is detected at cystoprostatectomy as in autopsies of men who died of other causes than PCa [23].
Sticky diagnosis bias [24], that is when PCa is erroneously registered as the primary cause of death in men who died of another cause, is a common bias resulting in an inflated estimate of PCa mortality as demonstrated in a previous study in PCBaSe [25]. In a structured review of 495 medical charts in a study frame of 5,543 men, 29% of men above age 85 with PCa and 21% of men below age 85 with low-risk PCa had PCa as adjudicated cause of death, despite little evidence of progression in terms of levels or increases in PSA, metastases on imaging, use of ADT, or progression to castration resistant PCa [25]. An even higher proportion of misclassification was found in a study in Norway [26]. Out of 328 men with PCa as adjudicated primary cause of death in The Cause of Death Register, a chart review could verify this in only 66% of the men. Similarly, in a recent study from Estonia, only 59% of deaths adjudicated to PCa were verified in a chart review [27].
In another study in PCBaSe, 90% of men who died of PCa had initiated ADT before date of death, showing that death from PCa is preceded by use of ADT as treatment for PCa progression in a large majority of men in Sweden [28].
In the present study, many men were old at date of death, a large majority had Gleason 6 or 7, and two thirds had not started ADT before date of death attributed to PCa in The Cause of Death Register. Furthermore, only 40% of men who died of PCa had bladder cancer recorded as contributing cause. Given the high fatality rate of bladder cancer, this could in some instances have been an error in the registration where ‘prostate cancer’ was erroneously substituted for ‘bladder cancer’ as primary cause of death. Based on these considerations, we argue that sticky-diagnosis bias was present in our study, and that the risk of PCa death based on data in The Cause of Death Register was substantially inflated.
Our results are in accordance with previous smaller studies with shorter follow-up [20, 21]. In a study in France of 931 men, no man had died of PCa after 25 months of follow-up, and in another study of 329 men, only one man died of PCa after 9 years of follow-up. In both studies, the rate of Gleason ≥ 8 was below 5%.
In line with previous studies, only 4% of men were diagnosed with Gleason ≥ 8 in our study, and for them, the risk of PCa death was three-fold higher than for men with Gleason 7 (13% vs. 4%). In men with a reasonably long life expectancy and Gleason ³ 8, PSA testing may be considered if early ADT is contemplated in the event of PSA progression.
In a previous study in PCBaSe, men with PCa who underwent radical prostatectomy had 1.4% risk of PCa death after 9 years of follow-up, and risk of death from other causes was 6% [29]. Thus, the risk of PCa death was quite similar as for men in the present study, but risk of death from other causes was 10-fold lower.
Our results are applicable to other Western countries since both the proportion of men diagnosed with incident PCa at cystoprostatectomy and the proportion of men with latent PCa detected at autopsy are around 30% in many Western countries [23]. There is also evidence that the proportion of men who erroneously have PCa adjudicated as cause of death in the official cause of death registration is high in many countries [25–27].
In conclusion, given the high prevalence of incidental PCa after cystoprostatectomy, the very low risk of PCa death, the high risk of death from bladder cancer and other causes, and the limited value of an early diagnosis of a relapse in older men who have undergone radical treatment [30], there is no rationale for PSA-testing in a large majority of men diagnosed with PCa after a cystoprostatectomy performed for bladder cancer.
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Elin Axén, Johan Styrke, Andreas Josefsson, Camilla Thellenberg, Hampus Nugin, Ingrida Verbiené, Stefan Carlsson, Anna Kristiansen, Mats Andén, Kimia Kohestani, Jon Kindblom, Thomas Jiborn, Olof Ståhl, Olof Akre, Eva Johansson, Magnus Törnblom, Fredrik Jäderling, Marie Hjälm-Eriksson, Lotta Renström Koskela, Jonas Hugosson, Ola Bratt, Erik Thimansson, Johan Stranne, Elin Trägårdh, Viktoria Gaspar, Fredrik Sandin, Petrus Stenson, Lena Pettersson, Mia Brus, Gustaf Hedström, Anna Hedström, Maria Moutran, Nina Hageman, Maria Nyberg, and patient representatives Hans Joelsson and Gert Malmberg.
Author contributions
- Conception and design: Scilipoti, Westerberg, Garmo, Liedberg, Stattin
- Acquisition of data: Scilipoti, Westerberg, Garmo
- Analysis and interpretation of data: Scilipoti, Westerberg, Garmo
- Drafting of the manuscript: Scilipoti, Westerberg, Liedberg, Stattin, Wilberg Orrason
- Critical revision of the manuscript for important intellectual content: Scilipoti, Westerberg, Garmo, Liedberg, Stattin, Wilberg Orrason
- Statistical analysis: Scilipoti, Westerberg, Garmo
- Obtaining funding: Stattin
- Supervision: Westerberg, Liedberg, Stattin
Context and relevance
One out of three men who undergo cystoprostatectomy for bladder cancer is diagnosed with prostate cancer (PCa) at examination of the specimen. Many of these men are PSA tested as part of their follow-up, but it is unclear if this is needed. After 15 years, the risk of death from PCa was well below 3%. These is no need for PSA testing during the follow-up for a large majority of these men.
References
- [1] Bell KJL, Del Mar C, Wright G, et al. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137(7):1749–1757. https://doi.org/10.1002/ijc.29538
- [2] Fahmy O, Khairul-Asri MG, Schubert T, et al. Clinicopathological features and prognostic value of incidental prostatic adenocarcinoma in radical cystoprostatectomy specimens: a systematic review and meta-analysis of 13,140 patients. J Urol. 2017;197(2):385–390. https://doi.org/10.1016/j.juro.2016.08.088
- [3] Bruins HM, Djaladat H, Ahmadi H, et al. Incidental prostate cancer in patients with bladder urothelial carcinoma: comprehensive analysis of 1,476 radical cystoprostatectomy specimens. J Urol. 2013;190(5):1704–1709. https://doi.org/10.1016/j.juro.2013.05.034
- [4] Thomas C, Giesswein A, Hainz M, et al. Concomitant gleason Score ≥7 prostate cancer is an independent prognosticator for poor survival in nonmetastatic bladder cancer patients undergoing radical cystoprostatectomy. Int Urol Nephrol. 2015;47(11):1789–1796. https://doi.org/10.1007/s11255-015-1110-1
- [5] Heidegger I, Oberaigner W, Horninger W, et al. High incidence of clinically significant concomitant prostate cancer in patients undergoing radical cystectomy for bladder cancer: a 10-year single-center experience. Urol Oncol. 2017;35(4):152.e1–5. https://doi.org/10.1016/j.urolonc.2016.11.004
- [6] Liedberg F, Kjellström S, Lind AK, et al. Swedish National Guidelines on Urothelial Carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand J Urol. 2022;56(2):137–146. https://doi.org/10.1080/21681805.2022.2041086
- [7] Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 1: early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand J Urol. 2022;56(4):265–273. https://doi.org/10.1080/21681805.2022.2094462
- [8] Bratt O, Carlsson S, Fransson P-A, et al. The Swedish national guidelines on prostate cancer, part 2: recurrent, metastatic and castration-resistant disease. Scand J Urol. 2022;56(4):278–284. https://doi.org/10.1080/21681805.2022.2093396
- [9] Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. Int J Epidemiol. 2015;45(1):73–82. https://doi.org/10.1093/ije/dyv305
- [10] Tomić K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51(1):101–111. https://doi.org/10.1016/j.ejca.2014.10.025
- [11] Tomić K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2014;54(2):158–163. https://doi.org/10.3109/0284186X.2014.939299
- [12] Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson Comorbidity Index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. https://doi.org/10.2147/CLEP.S282475
- [13] R Core Team. R: A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing, Vienna, Austria; 2022. [cited 14-12-2023] Available from: https://www.R-project.org/
- [14] Svenska Nationella Kvalitetsregistret för urinblåse-och urinvägscancer (SNRUBC). SNRUBC [Internet]. 2023 [cited 14-12-2023]. Available from: https://statistik.incanet.se/Urinblasecancer/
- [15] Cancerregistret. Socialstyrelsen [Internet]. 2023 [cited 14-12-2023]. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/cancerregistret/
- [16] Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. https://doi.org/10.1186/1471-2458-11-450
- [17] Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register – a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. https://doi.org/10.1080/02841860802247664
- [18] Fall K, Strömberg F, Rosell J, et al. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42(4):352–357. https://doi.org/10.1080/00365590802078583
- [19] Godtman R, Holmberg E, Stranne J, et al. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: acomparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45(4):226–232. https://doi.org/10.1080/00365590802078583
- [20] Pignot G, Salomon L, Neuzillet Y, et al. Clinicopathological characteristics of incidental prostate cancer discovered from radical cystoprostatectomy specimen: a multicenter French study. Ann Surg Oncol. 2013;21(2):684–690. https://doi.org/10.1245/s10434-013-3340-8
- [21] Packiam VT, Tsivian M, Avulova S, et al. Long-term outcomes of incidental prostate cancer at radical cystectomy. Urol Oncol. 2020;38(11):848.e17–22. https://doi.org/10.1016/j.urolonc.2020.05.018
- [22] Liedberg F, Anderson H, Bläckberg M, et al. Prospective study of transitional cell carcinoma in the prostatic urethra and prostate in the cystoprostatectomy specimen. Scand J Urol Nephrol. 2007;41(4):290–296. https://doi.org/10.1080/00365590601183576
- [23] Kimura T, Sato S, Takahashi H, et al. Global trends of latent prostate cancer in autopsy studies. Cancers. 2021;13(2):359. https://doi.org/10.3390/cancers13020359
- [24] Bergengren O, Westerberg M. Watch out for sticky diagnosis bias in older men with prostate cancer. Scand J Urol. 2022;56(5–6):365–366. https://doi.org/10.1080/21681805.2022.2124305
- [25] Orrason AW, Styrke J, Garmo H, Stattin P. Evidence of cancer progression as the cause of death in men with prostate cancer in Sweden. BJU Int. 2023;131(4):486–493. https://doi.org/10.1111/bju.15891
- [26] Löffeler S, Halland A, Weedon-Fekjær H, et al. High Norwegian prostate cancer mortality: evidence of over-reporting. Scand J Urol. 2018;52(2):122–128. https://doi.org/10.1080/21681805.2017.1421260
- [27] Innos K, Paapsi K, Alas I, et al. Evidence of overestimating prostate cancer mortality in Estonia: a population-based study. Scand J Urol. 2022;56(5–6):359–364. https://doi.org/10.1080/21681805.2022.2119274
- [28] Lycken M, Drevin L, Garmo H, et al. The use of palliative medications before death from prostate cancer: a Swedish population-based study with a comparative overview of European data. Eur J Cancer. 2018;88:101–108. https://doi.org/10.1016/j.ejca.2017.10.023
- [29] Thomsen FB, Garmo H, Brasso K, et al. Temporal changes in cause-specific death in men with localised prostate cancer treated with radical prostatectomy: a population-based, nationwide study. J Surg Oncol. 2021;124(5):867–875. https://doi.org/10.1002/jso.26579
- [30] van den Bergh RCN, van Casteren NJ, van den Broeck T, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016;69(5):802–820. https://doi.org/10.1016/j.eururo.2015.11.023