ORIGINAL RESEARCH ARTICLE
Study design and procedures in the incontinence post robot-assisted radical prostatectomy: anatomical and functional causes (IPA) – a prospective observational clinical trial
Katarina Koss Modiga,b, Rebecka Arnsrud Godtmana,b, Fredrik Langkildec,d, Marianne Månssona, Jonas Wallströmc,d and Johan Strannea,b
aDepartment of Urology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bDepartment of Urology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden; cDepartment of Radiology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; dDepartment of Radiology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden
ABSTRACT
Objective: To describe the study design and procedures of the incontinence post robot- assisted radical prostatectomy, anatomical and functional causes (IPA) trial. This trial aims to identify and study patient and procedure specific factors leading to urinary incontinence post robot-assisted laparoscopic radical prostatectomy (RALP).
Material and methods: The IPA study is a prospective, multicentre, open non-randomised surgical trial, including patients prior to RALP and registered on-line (ISRCTN67297115). IPA is administered from the Department of Urology at Sahlgrenska University Hospital, Gothenburg, Sweden. Patients undergo an anatomical and functional evaluation using magnetic resonance imaging (MRI), urodynamics including cystometry, pressure-flow and urethral pressure profile, and dynamic transrectal ultrasound prior to and 3 months after RALP. The incontinence data are gathered using patient reported outcome measure questionnaires. The primary endpoint is incontinence at 3 months after RALP, defined as need of any pad. The secondary endpoints are incontinence 12 months post RALP defined as need of any pad, and 3- and 12-months post RALP, defined as use of more than a safety pad.
Results: Until October 2023, 207 patients have been included of the stipulated 1,000, with an increasing rate of accrual. Out of these patients,187 have had a pre- and post-operative MRI and 177 have undergone pre- and post-operative urodynamics.
Conclusions: The design of the IPA study, together with promising accrual and coming multicentre inclusion, will hopefully result in the identification, and deeper understanding, of the various risk-factors for post-RALP incontinence. This could improve information and decision making regarding adequate treatment for patients with prostate cancer.
KEYWORDS: Urinary incontinence; prostate cancer; robot assisted radical prostatectomy; prospective clinical trial
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 156–161. https://doi.org/10.2340/sju.v59.40051.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 21 February 2024; Accepted: 3 September 2024; Published: 30 September 2024
CONTACT Johan Stranne johan.stranne@gu.se Department of Urology, Bruna Stråket 11B, 413 45 Gothenburg, Sweden
Introduction
The high incidence of prostate cancer (PC) diagnosed at a curative stage leads to a need for curative surgery [1]. Surgery for PC today is primarily provided as robot-assisted laparoscopic radical prostatectomy (RALP), with over 97% of radical prostatectomies in Sweden being robot assisted in 2022 [2]. RALP involves the removal of the entire prostatic gland and the seminal vesiculas, carrying a risk of damaging surrounding structures; for example, damage can occur at the neurovascular bundles, bladder neck and the sphincteric part of the urethra during apical dissection, resulting in side effects like erectile dysfunction and notably, post prostatectomy urinary incontinence (PPI) [3–5]. Between 4% and 30% of patients experience PPI 12 months post-surgery [6], indicating that this is a chronic problem for the men in question. Furthermore, incontinence severely diminishes the patient’s quality of life [7] and sometimes even leads to the patient regretting the choice of treatment [8].
The frequency of post-operative PPI varies between reporting clinics and surgeons, partly depending on varying definitions of PPI, but mainly indicating that specific aspects of each surgeon’s technique affect the risk [9–12]. There are a number of known risk factors for PPI, such as age, Body Mass Index (BMI), pre-operative membranous urethral length (MUL) measured on pre-operative magnetic resonance imaging (MRI) and degree of nerve-sparing surgery [13, 14]. Even functional measures such as membranous urethral closure pressure (MUCP) and functional urethral length (FUL), evaluated by urodynamics including urethral pressure profile (UPP), have been reported as possible predictors of PPI after RALP [15]. Of these factors, MUL is the most studied, and is suggested in the European Association of Urology (EAU) Guidelines for pre-operative risk assessment for post-RALP incontinence [16]. However, it is unclear exactly to what grade the MUL affects the absolute risk of PPI [17, 18] and how MUL co-varies with other known or unknown risk factors.
Some studies have attempted, in addition to measuring MUL, to anatomically describe the shape of the prostate apex, the pelvic floor and surrounding areas of the external sphincter using MRI, both pre- and post-operatively [19]. The pre-operative urethral appearance and length seem to play a significant role in post-operative continence [20]. Studies of UPP also show a decrease in MUCP and FUL after RALP, as well as a correlation between a higher MUCP and continence recovery. A comprehensive study, evaluating both MRI and UPP evaluation of patients prior to RALP, was therefore suggested already in 2012 [15]. To evaluate pelvic floor movements prior and after RALP, ultrasound imaging of the pelvic floor and the sphincter apparatus has also been used [21, 22].
The Swedish National Prostate Cancer Register (NPCR) is a quality registry with a capture rate of 98% [23]. The register contains data on patient and cancer characteristics, work-up and treatment. The registry also includes an electronic patient reported outcome measures questionnaire (ePROM), collected at baseline and at 3 and 12 months post-operatively.
To merge the methods previously used, and to utilise the existing data from NPCR, we initiated the ‘Incontinence post robot assisted radical prostatectomy, Anatomical and functional causes (IPA)’ study. In IPA, we prospectively collect data from pre- and post-operative MRI, transrectal ultrasound (TRUS) and urodynamics, from filming of the procedure, and specific patient, tumour and procedure data from NPCR. We then correlate this to pre- and post-operative continence data from the ePROM to pinpoint which patient and procedure-specific factors lead to PPI. Here, we present the IPA study, which to our knowledge, is the first multicentre study that combines all examination modalities, in combination with validated surgical, patient and continence data pre- and post-operatively. The aim of the trial is to identify patient and/or procedure specific factors leading to PPI, and to gain deeper knowledge on the mechanisms of these risk-factors.
Materials and methods
Trial design, endpoints, and participants
IPA is a prospective, multicentre, open non-randomised phase III surgical trial administered from the Department of Urology, Sahlgrenska University Hospital, aiming to include 1,000 patients. The study complies with international guidelines for the treatment of prostate adenocarcinoma (ethical approval number is Dnr 131-16) and the study protocol is registered in the ISRCTN-registry (ISRCTN67297115). The protocol was first approved in 2016 and updated in 2020 and 2023. Patients planned for RALP, fulfilling the inclusion criteria and none of the exclusion criteria presented in Table 1, are invited and, after providing informed consent, included. The key eligibility criteria are a continent patient diagnosed with prostatic cancer, elected for RALP. All subjects will adhere to the guidelines on treatment timing and receive any requisite post-operative care.
The primary objective of the trial is to identify patient and/or procedure specific factors leading to PPI. Secondary objectives are to gain further knowledge and understanding of how the actual mechanism of the risk-factors work; to investigate how RALP affects pelvic floor movement and its effect on PPI; to investigate how the function of the urinary bladder and urethra changes post RALP and its implications for PPI; to measure the changes of the prostate bed and its surroundings, with reference to pre-specified location of anatomical landmarks and its effect on PPI; to measure the changes in size, thickness, and location of surrounding organs (e.g., pelvic floor, rectum and remaining neurovascular bundles) and its effect on PPI.
For primary endpoint, we use incontinence, defined as need of any pad, at 3 months. However, as continence continues to improve up to 12 months post-operatively, and the definition of continence also differs between previously published trials, we also included 3 secondary endpoints: incontinence defined as any pad at 12 months and incontinence defined as need for more than a safety pad, at 3 and 12 months. Exact ePROM question used and definitions of the different cut-offs are displayed in Table 2.
Data collection and interventions
All patients undergo baseline and 3-month post-operative evaluations, including MRI, urodynamics with cystometry, a pressure-flow measure and UPP – and a dynamic TRUS. The RALP-procedures are recorded. A timeline of study visits and evaluations is depicted in Figure 1. Continence data are gathered from the NPCR ePROM questionnaire at baseline and at 3 and 12 months post-operatively. The questionnaire consists of 35 questions on general health, lower urinary tract, bowel and erectile function (see Appendix Table 1 for all questions used from ePROM), and has been face validated and tested by experts and prostate cancer survivors Patient and tumour baseline data, together with ePROM-data will be centrally collected from the NPCR and included in the electronic database. Relevant patient data not included in the NPCR, such as BMI, Eastern Cooperative Oncology Group (ECOG) performance status and American Society of Anaesthesiologists (ASA)-score, is continuously recorded in the central electronic database, prospectively at time of inclusion or collected from the local patient records. Magnetic resonance imaging, urodynamics and dynamic TRUS data are pseudo-anonymised and transferred for central storage and review.
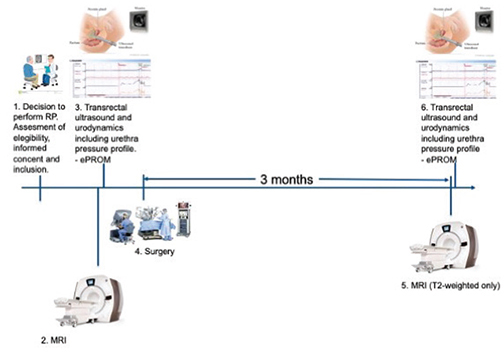
Figure 1. Timeline of study visits.
The management of data ensures the privacy of the subjects. Data will be analysed and reported only on group levels and no data will be linked to any individual. All records will be kept for 20 years from last inclusion.
Magnetic resonance imaging
Prior to surgery, most patients in the study will have undergone a diagnostic MRI within the previous year. If a diagnostic MRI is unavailable, we schedule one within the study before the surgery. All patients will also undergo a second MRI with only anatomical T2-weighted sequences, at 3 months post-operatively. The examinations are performed on a variety of MRI-scanners, using either a 1.5 or 3.0 Tesla, and with or without endorectal coil, but must meet the technical requirements according to PI-RADS v2.1 [24]. The MRI examinations will be saved and centrally reviewed.
All measurements on MRI will be performed by a radiologist with more than 5 years of prostate MRI reading experience. T2-weighted sequences will be used for measurements.
Urodynamics
Urodynamics, is carried out in line with standard clinical practices at local urological departments. It follows good urodynamic practice [25] and includes cystometry, a pressure flow examination and UPP. The tests are conducted at baseline and 3 months after RALP. All urodynamic curves are saved on paper or electronically, pseudo-anonymised and centrally reviewed. During both visits (pre- and post-operatively), the patients are checked that they have complete the ePROM on-line.
Dynamic transrectal ultrasound
During the time of urodynamics, a dynamic TRUS-examination is performed and recorded, where the patient lies on his back relaxed and performing Kegel exercise. The focus of the exam is the apical part of the prostate and area corresponding to MUL and the pelvic floor. The examinations are recorded, pseudo-anonymised and centrally reviewed.
Surgical recording
All RALP-procedures are recorded, pseudo-anonymised and centrally reviewed.
All patient and procedure specific factors from local records, NPCR, MRI, urodynamics, TRUS and surgery recordings in the study, and details on how they are assessed, are listed in Appendix Table 1. All measurements from the central review will be entered directly into the electronic database.
Statistics
To avoid difficulties with potential collinearity between variables, which might prevent correct interpretation of the coefficients, the following strategy will be followed. Firstly, publications on incontinence and risk factors on related patient’s groups will be considered to determine potential risk factors in the present study. Secondly, variables will be grouped by their correlation structure using variable clustering. Clinical knowledge will then be used to refine the clusters. For each cluster, a single representative, or a combination of the variables, for instance its first principal component, will be used in the regression modelling.
Membranous urethral length, a known risk-factor measured on pre-operative MRI, is used for power calculation. The study is powered to detect 10% increase in risk of incontinence at 3 months (defined as any pad use), per mm shortening of MUL, with 80% power if the risk of incontinence is approximately 20%, giving the estimated sample size for a one-sided test of approximately 800 patients for significance level 5%. To avoid loss of power, imputation of missing data in the potential risk factors will be performed by means of multiple imputation by chained equations (MICE). We estimate a drop-out and missing data rate of 10%. To account for this, and to allow for analysis of other known or unknown secondary parameters and potential risk-factors, we target a sample size of 1,000 patients. Furthermore, a sensitivity analysis of complete cases will be performed.
All analyses of factors affecting both the primary and secondary endpoints will be performed after completion of subject enrolment. All subjects with 3 months ePROM data on continence will be included in main analyses and all subjects with 3- and/or 12-months PROM data on continence included in secondary analyses.
All continuous variables will be described using median and interquartile range (IQR). Categorical variables will be described using frequencies and proportions. Both univariable and multivariable poisson regression analyses will be used to identify patient- and/or procedure-specific factors leading to urinary incontinence according to each endpoint [26].
The statistical methodology will be detailed in a Statistical Analysis Plan before the start of the analysis.
Results
Between December 2017 and October 2023, 207 patients have been included in the study at the primary study centre. During Q3–Q4 2024, the inclusion is starting at three participating centres outside Gothenburg.
Of the 207 included patients, 204 underwent surgery. All patients had pre-operative MRI but 17 did not have a post-operative MRI, of whom 14 chose to discontinue participation in the study and 3 because of COVID-19 restrictions (Figure 2). The primary reason for discontinuation was ‘lack of time’, and most of those who spontaneously provided a reason described themselves as being continent. Twenty five patients did not undergo pre-operative urodynamics and an additional four did not perform post-operative ditto (Figure 2). The reasons were similar for pre- and post-operative dropout from urodynamics, that is catheter insertion problem, discomfort, technical problems and COVID-19 restrictions. All patients, who discontinued the study, consented for already gathered data to be used in the study. After a logistics change, where the pre-operative cystometry/TRUS was coordinated with the pre-surgery consultation with the anaesthesiologist, and the post-operative MRI, urodynamics and TRUS was coordinated to take place on one occasion, the drop-out from the study lowered significantly.
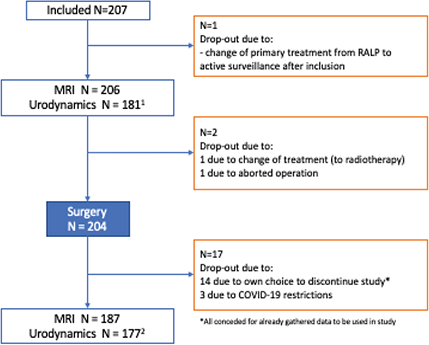
Figure 2. Flow chart of study participants, drop-out rates and reasons why.
Additional drop-out (during urodyamics, not MRI):
1 N=25: 8 due to problem with catheter insertion; 4 due to technical issues with equipment; 7 due to COVID-19-restriction; 4 due to failing logistics; 2 due to discomfort of examination.
2 N=10: 3 due to problem with catheter insertion; 6 due to discomfort of examination; 1 due to failing logistics
The study started at a single centre to test logistics and willingness of patients to participate, with the aim of expanding to more clinics if possible. The COVID-19 pandemic delayed the expansion. During autumn of 2024, the departments of Urology in Malmö, Stockholm and Skövde are joining the study, with start of Q3 to Q4.
There have been no adverse events because of the interventions.
Discussion
Despite development of surgical technique, PPI is still a significant problem for a number of men after RALP. Furthermore, as a majority is cured from their PC and have a long-life expectancy, the impact on the quality of life is high [26]. We know some of the risk-factors causing this bothersome complication, but we have far from the whole picture. There have been several studies illuminating different aspects of RALP and their respective potential effect on the risk of PPI.
The Swedish public healthcare system, together with the high degree of coverage of the NPCR, enables ready access to validated data of outcomes after treatment, as well as to a number of risk-factors. By combining this with previously suggested evaluation modalities, the IPA study is an attempt to take an overall grip to identify, and if possible, quantify the majority of patient- and procedure-specific risk-factors behind PPI.
Since the start of inclusion in the IPA study, we have included 207 patients. A limiting factor for inclusion in the study is its design involving invasive procedures such as urodynamics and TRUS at two different time points together with an extra MRI examination. Among patients asked to participate, some have expressed a fear of discomfort during these examinations. Initially, the need for multiple additional hospital visits for various led to dropouts. The inclusion rate was further limited by the varying availability of examinations urodynamics at the hospital and the challenges posed by the COVID-19 pandemic. There is also an issue of dropout during the study, the highest rate being among patients planned for post-operative examinations. Some patients choose to discontinue participation when they are continent after RALP. The need of extra visit 3 months post-surgery might also influence the dropout rates, especially since many participants are of working-age and live far from the study centre. Initially, we estimated a 10% dropout rate – a number which was initially exceeded. However, by better informing patients about the investigations and streamlining appointments, consolidating most cystometry and MRI procedures into just one additional hospital visit, the dropout rate was reduced significantly. This experience will be implemented in the planning of logistics at the additional sites joining. Depending on the nature of the variables, some degree of missing data is to be expected. To avoid loss of power, we will also apply imputation of missing data of potential risk factors by means of MICE. We will also perform a sensitivity analysis of complete cases. With the additional three centres, we expect an accelerated inclusion rate, bringing us closer to our target of 1,000 included patients, with at least 800 being evaluable. The inclusion started in December 2017 and is planned to be completed by Q4 2026.
In this study, we use TRUS to measure pelvic floor movement as compared to previously described trans-perineal ultrasound (TPUS). The use of TPUS has been described by Mungovan et al.in the setting of post-operative follow up. Trans-perineal ultrasound has the advantage of not affecting the pelvic floor and its movement at all, as the transducer is not inserted in the rectum but put outside to the perineal area. However, TRUS is a method well familiar to all urologists and is routinely used pre-operatively to measure the size of the prostate, to guide biopsies and to register potential variations in the normal anatomy. In order not to introduce a novel ultrasound modality for the investigating urologist, we have therefore chosen to use this for investigating the pelvic floor movement. After performing 400 pre or post-operative examinations, and as the pubic bone is the reference point for the pelvic floor movement, we believe that the probe affects the movement minimally.
The study’s major strengths include its prospective, multicentre design, its size and its comprehensive approach to evaluating both known and unknown risk-factors for PPI. The study design enables evaluation of inherent patient factors, including physiological, anatomical and functional aspects, and it also assesses many standardised procedure-specific factors. This, to our knowledge, is unique. The major limitation of the study is a shortage of solid knowledge from previous studies, on how to interpret the results of the aggregated investigations made in the study in the context of PPI. There may also, given the invasive nature of some of the study procedures, be a risk of selection bias which may impact who consents to the study in, unknown ways.
Prospective randomised clinical trials are seen by many as the fundamental ideal for acquiring knowledge, especially concerning effects, wanted or unwanted, of a medical intervention. However, while such trials are often well-suited for comparing a pharmaceutical substance to placebo or one medical procedure to another, they may not be ideal for exploring which patient factors or surgical procedure elements affect the risk of PPI and to what extent. Although we are aware of some factors affecting PPI, we lack a comprehensive understanding of all the potential co-varying factors influencing this outcome. If we successfully chart the influencing risk-factors, our next step could be designing randomised interventional trials to reduce the impact of these factors.
The IPA trial’s design, combined with its promising accrual as new sites join and with the oversight of the national quality control NPCR, will hopefully result in detailed and valuable information on the various risk factors of post-operative PPI. With more thorough knowledge of these risk-factors, we will hopefully be able to advise patients more effectively, informing them about their specific risk and benefit, before scheduling a potentially harmful procedure.
Acknowledgments
We thank registered nurses and urotherapists Marianne Ferring and Anna Martinsson De Cardenas, at the Department of Urology, Sahlgrenska University Hospital, for performing urodynamic examinations and their invaluable help in executing the study.
ORCID
Katarina Koss Modig https://orcid.org/0000-0002-6396-0645
Rebecka Arnsrud Godtman https://orcid.org/0000-0003-2061-6550
Fredrik Langkilde https://orcid.org/0000-0002-8706-4036
Marianne Månsson https://orcid.org/0000-0002-1460-4062
Jonas Wallström https://orcid.org/0000-0002-4453-6718
Johan Stranne https://orcid.org/0000-0002-4295-6524
References
- [1] Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. https://doi.org/10.3322/caac.21660
- [2] Database on in-house surgery [Internet]. 2024 [cited 02-01-2024]. Available from: https://sdb.socialstyrelsen.se/if_ope/val.aspx
- [3] Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68(2):216–225. https://doi.org/10.1016/j.eururo.2015.02.029
- [4] Cazzaniga W, Godtman RA, Carlsson S, et al. Population-based, nationwide registration of prostatectomies in Sweden. J Surg Oncol. 2019;120(4):803–812. https://doi.org/10.1002/jso.25643
- [5] Du Y, Long Q, Guan B, et al. Robot-assisted radical prostatectomy is more beneficial for prostate cancer patients: a system review and meta-analysis. Med Sci Monit. 2018;24:272–287. https://doi.org/10.12659/MSM.907092
- [6] Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62(3):405–417. https://doi.org/10.1016/j.eururo.2012.05.045
- [7] Wallerstedt A, Carlsson S, Nilsson AE, et al. Pad use and patient reported bother from urinary leakage after radical prostatectomy. J Urol. 2012;187(1):196–200. https://doi.org/10.1016/j.juro.2011.09.030
- [8] van Stam MA, Aaronson NK, Bosch J, et al. Patient-reported outcomes following treatment of localised prostate cancer and their association with regret about treatment choices. Eur Urol Oncol. 2020;3(1):21–31. https://doi.org/10.1016/j.euo.2018.12.004
- [9] Nyberg M, Sjoberg DD, Carlsson SV, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the Swedish LAPPRO trial. BJU Int. 2021;127(3):361–368. https://doi.org/10.1111/bju.15238
- [10] Carlsson S, Berglund A, Sjoberg D, et al. Effects of surgeon variability on oncologic and functional outcomes in a population-based setting. BMC Urol. 2014;14:25. https://doi.org/10.1186/1471-2490-14-25
- [11] Clements MB, Gmelich CC, Vertosick EA, et al. Have urinary function outcomes after radical prostatectomy improved over the past decade? Cancer. 2022;128(5):1066–1073. https://doi.org/10.1002/cncr.33994
- [12] Godtman RA, Persson E, Cazzaniga W, et al. Association of surgeon and hospital volume with short-term outcomes after robot-assisted radical prostatectomy: nationwide, population-based study. PLoS One. 2021;16(6):e0253081. https://doi.org/10.1371/journal.pone.0253081
- [13] Lardas M, Grivas N, Debray TPA, et al. Patient- and tumour-related prognostic factors for urinary incontinence after radical prostatectomy for nonmetastatic prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2022;8(3):674–689. https://doi.org/10.1016/j.euf.2021.04.020
- [14] Steineck G, Bjartell A, Hugosson J, et al. Degree of preservation of the neurovascular bundles during radical prostatectomy and urinary continence 1 year after surgery. Eur Urol. 2015;67(3):559–568. https://doi.org/10.1016/j.eururo.2014.10.011
- [15] Dubbelman YD, Bosch JL. Urethral sphincter function before and after radical prostatectomy: systematic review of the prognostic value of various assessment techniques. Neurourol Urodyn. 2013;32(7):957–963. https://doi.org/10.1002/nau.22355
- [16] EAU – EANM – ESTRO – ESUR – ISUP – SIOG guidelines on prostate cancer [Internet]. 2023 [cited 02-02-2024]. Available from: https://uroweb.org/guidelines/prostate-cancer
- [17] van Dijk-de Haan MC, Boellaard TN, Tissier R, et al. Value of different magnetic resonance imaging-based measurements of anatomical structures on preoperative prostate imaging in predicting urinary continence after radical prostatectomy in men with prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2022;8(5):1211–1225. https://doi.org/10.1016/j.euf.2022.01.015
- [18] Tutolo M, Rosiello G, Stabile G, et al. The key role of levator ani thickness for early urinary continence recovery in patients undergoing robot-assisted radical prostatectomy: a multi-institutional study. Neurourol Urodyn. 2022;41(7):1563–1572. https://doi.org/10.1002/nau.25001
- [19] Lee SE, Byun SS, Lee HJ, et al. Impact of variations in prostatic apex shape on early recovery of urinary continence after radical retropubic prostatectomy. Urology. 2006;68(1):137–141. https://doi.org/10.1016/j.urology.2006.01.021
- [20] Paparel P, Akin O, Sandhu JS, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009;55(3):629–637. https://doi.org/10.1016/j.eururo.2008.08.057
- [21] Mungovan SF, Sandhu JS, Akin O, et al. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: a systematic review and meta-analysis. Eur Urol. 2017;71(3):368–378. https://doi.org/10.1016/j.eururo.2016.06.023
- [22] Mungovan SF, Carlsson SV, Gass GC, et al. Preoperative exercise interventions to optimize continence outcomes following radical prostatectomy. Nat Rev Urol. 2021;18(5):259–281. https://doi.org/10.1038/s41585-021-00445-5
- [23] Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of the national prostate cancer register of Sweden. Acta Oncol. 2015;54(2):158–163. https://doi.org/10.3109/0284186X.2014.939299
- [24] Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–351. https://doi.org/10.1016/j.eururo.2019.02.033
- [25] Rosier P, Schaefer W, Lose G, et al. International continence society good urodynamic practices and terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36(5):1243–1260. https://doi.org/10.1002/nau.23124
- [26] Guangyong Zou. A modified poisson regression approach to prospective studies with binary data Am J Eoidemiol. 2004 Apr 1;159(7):702-6. PMID: 15033648 https://doi.org/10.1093/aje/kwh090
- [27] Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367(7):595–605. https://doi.org/10.1056/NEJMoa1201637