ORIGINAL RESEARCH ARTICLE
Reduction of lower urinary tract symptoms in prostate cancer patients treated with robot assisted laparoscopic prostatectomy
Lars Fredrik Qvigstada*, Lars Magne Eria,b, My Diep Lien MDc, Sophie Dorothea Fossåb,d, Kirsti Aasb,e and Viktor Bergea,b
aDepartment of Urology, Oslo University Hospital, Oslo, Norway; bInstitute for Clinical Medicine, University of Oslo, Oslo, Norway; cOslo Hospital Service, Research Support, Oslo University Hospital, Oslo, Norway; dDepartment of Oncology, Oslo University Hospital, Oslo, Norway; eAkershus University Hospital, Lørenskog, Norway
ABSTRACT
Problem: The aim of this study was to evaluate the change in lower urinary tract symptoms (LUTS) in patients treated with robot assisted laparoscopic prostatectomy (RALP) and to assess factors that may predict a reduction of LUTS after RALP and how this influences quality of life (QoL).
Materials and method: In our institutional prospective research registry, 1,935 patients operated in the period between 2009 and 2021 with baseline- and 12-month EPIC-26 questionnaire were eligible for the study. SF-12 data estimating general QoL were also analyzed. A domain summary score was constructed from the four questions concerning obstructive/irritative voiding symptoms, and transformed linearly to a 0–100 scale with higher scores representing less symptoms. A change of 6 points or more was considered Meaningful Clinical Differences (MCD). Two summary scores were calculated from the SF-12 – a mental component score (MCS-12) and a physical component score (PCS-12). Multi variable regression was used to estimate covariates associated with postoperative change in MCD, MCS-12 and PCS-12.
Results: Mean LUTS-score showed an increase of 4,3 points 12-months post-RALP. A total of 50.4% of patients achieved MCD. In multivariate logistic regression, preoperative LUTS was statistically significant associated with MCD. Reduction of LUTS was associated with improved mean score of MCS-12 and PCS-12.
Discussion and conclusion: Along with information about risk for urinary incontinence after RALP, patients with LUTS at baseline should be informed that these symptoms may be reduced after RALP. In our study, this LUTS reduction was associated with better general QoL.
KEYWORDS: Prostate cancer; radical prostatectomy; LUTS; EPIC-26; SF-12
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 121–125. https://doi.org/10.2340/sju.v59.40070.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 8 February 2024; Accepted: 14 May 2024; Published: 18 June 2024
CONTACT: Lars Fredrik Qvigstad larqvi@ous-hf.no Department of Urology, Oslo University Hospital, Radiumhospitalet, Postboks 4953 Nydalen, 0424 Oslo, Norway
Introduction
Prostate cancer (PCa) and benign prostatic hyperplasia (BPH) usually occur in men of advanced age and frequently coexist [1]. BPH may cause lower urinary tract symptoms (LUTS) and increase serum PSA levels. LUTS is not associated with an increased risk of PCa [2]. The prevalence of BPH increases with age, reaching 50% – 60% for men in their 60s [3]. Men suffering from LUTS often seek medical advice and undergo PSA testing as part of the clinical evaluation [1, 4, 5]. LUTS often reduce patients’ quality of life (QoL) due to storage- (e.g. urgency, nocturia) and/or voiding-symptoms (e.g. intermittency, weak voiding stream) [6, 7] and can even increase the mortality risk [8].
Men with non-metastatic PCa and at least 10 years life expectancy may be offered curative treatment with either radical prostatectomy (RP), external beam radiotherapy (EBRT) or brachytherapy (BT). Neither EBRT nor BT reduce the prostatic obstruction, and moderate/severe LUTS is regarded as a contraindication to BT [9].
Data about changes in LUTS after local PCa treatment, assessed by patient-reported outcome measures, is seldom reported in PCa studies [10]. The impact of changes in urinary function, i.e. LUTS on QoL after robot assisted laparoscopic prostatectomy (RALP) is underreported as well [11]. Preoperative information offered to the patients is mainly focused on the risk of postoperative urinary incontinence and erectile dysfunction. In the present study, we sought to address the impact of RALP on LUTS in PCa patients and assess predictors for reduction of LUTS after RALP. We also wanted to assess the impact of LUTS on general QoL.
Patients and methods
Clinical data are consecutively recorded in our institutional Research Registry of Prostate Cancer. To evaluate patient urinary function after RALP, we used the self-administered questionnaire EPIC 26 (Expanded PCa Index-Composite). It is mailed to the patients before surgery (baseline) and at 3, 12 and 36 months after RALP. EPIC-26 [12, 13] contains 26 items which constitute five domains: Urinary Incontinence, Urinary Obtructive/Irritative, Bowel, Sexual, and Hormonal. Response options for each EPIC item form a Likert scale, and multi-item scores are transformed linearly to a 0–100 scale, with higher scores representing better QoL. Use of EPIC-26 is the recommended PCa-specific Patient Reported Outcomes Measures (PROMs) instrument in National Cancer Institute-sponsored clinical trials [12] and is recommended by the International Consortium for Health Outcomes Measurement [13]. The Norwegian version of EPIC-26 has shown acceptable reliability and validity for assessment of adverse effects after treatment of non-metastatic PCa [14].
Urinary irritative/obstructive symptoms are described by four questions: How big a problem, if any, has each of the following been for you during the last 4 weeks? 1. Pain or burning on urination? 2. Bleeding with urination? 3. Weak urine stream or incomplete emptying? 4. Need to urinate frequently during the day?
In analysis ‘change of LUTS score’ was defined as the LUTS score at 12 months postoperatively minus the LUTS score at baseline. The minimum Meaningful Clinical Differences (MCD) for the urinary irritative-obstructive domain is estimated to a range of 5–7 points [15]. An increase of 6 points or more for change of LUTS score was considered MCD.
Nervesparing (NS) was dichotomized into no NS and NS. Preoperative risk group stratification was based on the European Association of Urology (EAU) classification [16] and dichotomized into low/intermediate and high risk groups.
Information about comorbidity was obtained from questions included in the questionnaire about presence in the patient’s history of the following diagnoses: diabetes, coronary heart disease, stroke, pulmonary disease, neurological disease, depression and renal disease. Comorbidity was dichotomized into no comorbidity and comorbidity (one or more of the diagnoses listed above).
Prostate volume (PV) was calculated on preoperative MRI or transrectal ultrasound.
General QOL was assessed by SF-12 (short form health survey), completed by patients between 2009 and 2016. SF-12 is a health survey yielding two summary scores assessing physical component score function (PCS-12) and mental component score well-being (MCS-12) [17]. The Norwegian population mean PCS-12 and MCS-12 scores were 50,3 and 50,6, respectively [18].
Patients who received adjuvant or salvage radiation therapy or androgen deprivation therapy (ADT) within 12 months after RALP were excluded from the analyses.
Statistics
Patient characteristics are described as mean with standard deviation (SD) and range for continuous data and frequencies for categorical data. Differences between paired total scores were examined by using one-sample t-test. A change in the total score of LUTS of at least 6 points from baseline to 12 months is considered as MCD in LUTS. Predictors for reduction of LUTS after RALP were assessed using logistic regression. Association between independent variables of interest and total scores of mental and physical components were studied by performing linear regression analyses. For the analyses of linear regression, the goodness of fit was checked by plotting the predicted values vs standardized residuals and calculating the coefficient of determination (R2). The R2 indicates the proportion of variance in the dependent variable associated with the independent variables and ranges between 0 and 1. A large value of R2 indicates a large variation explained by the model and good fit to the data. For logistic regression analyses, the Hosmer-Lemeshow test and value of Nagelkerke’s R2 were used to check the goodness of fit [19]. A value of R2 < 0.01 from a univariable analysis of regression was considered as independence (i.e. no association) between the outcome and independent variable. Thereby the independent variable was omitted/excluded from further analysis.
P-value less than 0.05 was considered statistically significant. All statistical analyses were performed by using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA).
Results
During the study period, 3,851 patients underwent RALP and were eligible for the study. Of these patients, 2,811 patients (73%) signed informed consent for inclusion in our Research Registry of Prostate Cancer. All of these patients were offered EPIC-26 and 2,230 patients (79%) returned the questionnaire both at baseline and at 12 months follow-up. Within 12 months postoperatively, 295 patients had received postoperative radiation therapy and/or ADT and were excluded from the study. Hence, this study comprised 1,935 patients with characteristics depicted in table 1. We found a mean increase in LUTS score (clinical improvement) of 4,3 points (Table 2, Figure 1), using the 4 items recommended in the original coding instructions for EPIC 26 [15]. About half of the patients (50.4%) achieved a change in LUTS score corresponding with MCD (Figure 2) (P = 0.8).
| EPIC 26 | N = 1,935 | P | |||
| Mean | SD | n | % | ||
| 12 months postoperative LUTS score, mean (SD) | 88.9 | 13 | <0.001 | ||
| Changea from baseline, mean (SD) | +4.3 | 15 | |||
| 12 months postoperative Urinary Incontinence summary score, mean (SD) | 73.3 | 27 | <0.01 | ||
| Changea from baseline, mean (SD) | –20.0 | 27 | |||
| 12 months postoperative Use of ≥ 2 pads per day (item 3), N (%) | 250 | 13 | |||
| SF-12 | n = 911 | ||||
| 12 months postoperative Physical Component Summary (PCS) score, mean (SD) | 51.2 | 8 | <0.01 | ||
| aPCS Change from baseline, mean (SD) | +1.7 | 7 | |||
| 12 months postoperative Mental Component Summary (MCS) score, mean (SD) | 54.4 | 9 | <0.01 | ||
| aMCS change from baseline | –1.5 | 9 | |||
| aScore difference between baseline and 12 months after RP: worsening (–); improvement (+). MCS: mental component score; PCS: physical component score; LUTS: lower urinary tract symptoms; RALP: robot assisted laparoscopic prostatectomy. | |||||
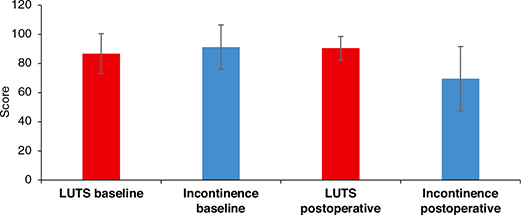
Figure 1. Mean Summary score of LUTS og urinary incontinence score with standard deviation. Higher scores denote less LUTS and incontinence.
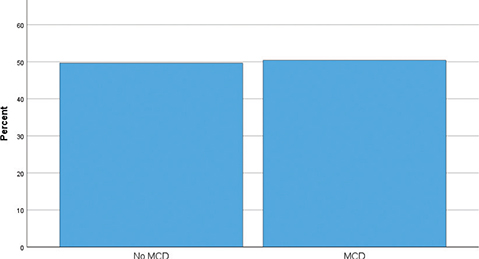
Figure 2. Percentage of patients with change of lower urinary tract symptoms (LUTS) score similar or higher than Meaningful Clinical Differences (MCD). Six points improvement of LUTS score were considered the lower limit for MCD.
At 12 months post-RALP, 13% of the patients reported daily use of two pads or more (Table 2). Urinary continence decreased at 12 months post-RALP as shown by a decrease in mean summary urinary incontinence score by 21 points (Table 2, Figure 1).
In univariable logistic regression analyses, the LUTS score at baseline had a Nagelkerke’s R2 = 0.41, indicating a strong relationship with MCD at 12 months post-RALP (Table 3). Independent variables such as NS, comorbidity and EAU risk group had Nagelkerke’s R2 < 0.01, indicating that very little of the variance in the dependent variable MCD can be explained by these binary independent variables (data not shown). In multivariable logistic regression analyses, LUTS score at baseline and prostate volume retained its statistical significance, whereas age did not (Table 3).
A total of 911 patients returned SF-12 questionnaire both at baseline and 12 month post-RALP. For assessment of general QoL, analyses of SF-12 data showed improvement of MCS-12 score and deterioration of PCS-12 score at 12 months postoperatively (Table 2). Reduced LUTS and less urinary incontinence at 12 months were significantly associated with better mental and physical health 12 months postoperatively (Tables 4 and 5).
Discussion
In this study we have shown that patients undergoing RALP had a reduction of LUTS at 12 months postoperatively, and the reduction was associated with the degree of preoperative LUTS and preoperative prostate volume. Age was not predictive of LUTS reduction. Moreover, the reduction of LUTS symptoms was associated with better general QoL.
We are not aware of other studies of similar sample size using EPIC 26 for evaluating changes in LUTS in patients undergoing RALP. Our study corroborates findings by Leyh‑Bannurah [20] in 5,506 RALP patients using International Prostate Symptom Score (IPSS) at baseline and 12 months after RALP. They reported that a higher preoperative LUTS burden (severe vs. moderate) was independent predictors of LUTS reduction after RALP. In an older study by Masters et al. [21] examining 125 RALP patients with urinary flowmetry and IPSS score at baseline and postoperatively, 38% and 56% of the patients had bladder outlet obstruction (defined by a flow rate of ≤10 mL/s) and moderate/severe symptoms (IPSS ≥ 8) before surgery, respectively. At 20-month follow-up, the median flow rate increased to 24 mL/s and the proportion of patients with IPSS ≥ 8 decreased to 14%.
In our study, the association between PV and MCD was weak, indicated by Nagelkerke’s R2 of 0.02. This finding is in line with the current understanding of LUTS as a disorder often unrelated to prostate enlargement [3].
The findings by SF-12 data showing association between reduction of LUTS and better general QoL have also been shown by others [22]. Our SF-12 data also reveal an assosciation between urinary incontinence and mental- and physical health. When constructing a summary score for urinary function consisting of all 8 questions in the EPIC-26 urinary domains, hence includes both LUTS- and incontinence score, Berge et al. [23] found that better urinary function was associated with better mental health.
Strengths of this study are relatively large patient population with a relatively high response rate to the EPIC-26 questionnaire data at baseline and 12 months post RALP. Complementary clinical data gave the opportunity to analyse relevant predictors. One limitation in our study was the lack of urodynamic examination pre- and postoperatively which is better suited to confirm improvement of urinary flow. Another limitation is that the use of IPSS could have documented more detailed changes in LUTS compared to the more coarse EPIC-26 questionnaire, which also include items not so relevant for LUTS. We were not able to find studies with a comparison between EPIC-26 and IPSS. In a paper by Vertosick et al. [24], the authors concluded that no comparison was possible due to differences in the domains addressed by these questionnaires.
Conclusion
PCa patients experience a reduction in LUTS after RALP. Along with information about the risk for urinary incontinence, patients with LUTS should be informed that these symptoms may be reduced after RALP. In our study, this LUTS reduction was associated with better general QoL.
ORCID
Lars Fredrik Qvigstad  https://orcid.org/0000-0002-7615-1695
https://orcid.org/0000-0002-7615-1695
References
- [1] Kim JH, Ha YS, Jeong SJ, et al. Impact of robot-assisted radical prostatectomy on lower urinary tract symptoms and predictive factors for symptom changes: a longitudinal study. Urology. 2013 Apr;81(4):787–93. https://doi.org/10.1016/j.urology.2012.12.038
- [2] Chandra Engel J, Palsdottir T, Aly M, et al. Lower urinary tract symptoms (LUTS) are not associated with an increased risk of prostate cancer in men 50–69 years with PSA ≥3 ng/ml. Scand J Urol. 2020 Feb;54(1):1–6. https://doi.org/10.1080/21681805.2019.1703806
- [3] Chapple CR, Wein AJ, Abrams P, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol. 2008 Sep;54(3):563–9. https://doi.org/10.1016/j.eururo.2008.03.109
- [4] Latz I, Weber M, Korda R, et al. Lower urinary tract symptoms in relation to region of birth in 95,393 men living in Australia: the 45 and up study. World J Urol. 2013 Jun;31(3):673–82. https://doi.org/10.1007/s00345-012-0937-7
- [5] Walz J, Suardi N, Hutterer GC, et al. Lower urinary tract symptoms affect one-third of men in a prostate cancer screening population. J Endourol. 2008 Feb;22(2):369–76. https://doi.org/10.1089/end.2007.0135
- [6] Kupelian V, Wei JT, O’Leary MP, et al. Nocturia and quality of life: results from the Boston area community health survey. Eur Urol. 2012 Jan;61(1):78–84. https://doi.org/10.1016/j.eururo.2011.05.065
- [7] Geraerts I, Van Poppel H, Devoogdt N, et al. Prospective evaluation of urinary incontinence, voiding symptoms and quality of life after open and robot-assisted radical prostatectomy. BJU Int. 2013 Nov;112(7):936–43. https://doi.org/10.1111/bju.12258
- [8] Åkerla J, Pesonen JS, Pöyhönen A, et al. Impact of lower urinary tract symptoms on mortality: a 21-year follow-up among middle-aged and elderly Finnish men. Prostate Cancer Prostatic Dis. 2019 May;22(2):317–23. https://doi.org/10.1038/s41391-018-0108-z
- [9] Locke J, Ellis W, Wallner K, et al. Risk factors for acute urinary retention requiring temporary intermittent catheterization after prostate brachytherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2002 Mar 1;52(3):712–19. https://doi.org/10.1016/S0360-3016(01)02657-8
- [10] Whiting PF, Moore TH, Jameson CM, et al. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: a systematic review. BJU Int. 2016 Aug;118(2):193–204. https://doi.org/10.1111/bju.13499
- [11] Dommer L, Birzele JA, Ahmadi K, et al. Lower urinary tract symptoms (LUTS) before and after robotic-assisted laparoscopic prostatectomy: does improvement of LUTS mitigate worsened incontinence after robotic prostatectomy? Transl Androl Urol. 2019 Aug;8(4):320–8. https://doi.org/10.21037/tau.2019.06.24
- [12] Chen RC, Chang P, Vetter RJ, et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Inst. 2014 Jul;106(7):dju132. https://doi.org/10.1093/jnci/dju132
- [13] Martin NE, Massey L, Stowell C, et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol. 2015 Mar;67(3):460–7. https://doi.org/10.1016/j.eururo.2015.08.016
- [14] Fossa SD, Storas AH, Steinsvik EA, et al. Psychometric testing of the Norwegian version of the Expanded Prostate Cancer Index Composite 26-item version (EPIC-26). Scand J Urol. 2016 Aug;50(4):280–5. https://doi.org/10.3109/21681805.2016.1163617
- [15] Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015 Jan;85(1):101–5. https://doi.org/10.1016/j.urology.2014.08.044
- [16] Cornford P, Tilki D, van den Bergh RCN, Briers E, et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer. Edn presented at the EAU Annual Congress Paris 2024, ISBN 978-94-92671-23-3, EAU Guidelines Office, Arnhem, The Netherlands, 2024.
- [17] Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy. 1997 Jan;2(1):14–18. https://doi.org/10.1177/135581969700200105
- [18] Gandek B, Ware J, Aaronson N, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. J Clin Epidemiol. 1998;51:1171–8. https://doi.org/10.1016/S0895-4356(98)00109-7
- [19] Nagelkerke N. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2. https://doi.org/10.1093/biomet/78.3.691
- [20] Leyh-Bannurah SR, Wagner C, Schuette A, et al. Improvement of quality of life and symptom burden after robot-assisted radical prostatectomy in patients with moderate to severe LUTS. Sci Rep. 2021 Aug 18;11(1):16757. https://doi.org/10.1038/s41598-021-95525-2
- [21] Masters JG, Rice ML. Improvement in urinary symptoms after radical prostatectomy: a prospective evaluation of flow rates and symptom scores. BJU Int. 2003 Jun;91(9):795–7. https://doi.org/10.1046/j.1464-410X.2003.04231.x
- [22] Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006 Oct;68(4):804–9. https://doi.org/10.1016/j.urology.2006.04.019
- [23] Berge V, Berg RE, Hoff JR, et al. A prospective study of transition from laparoscopic to robot-assisted radical prostatectomy: quality of life outcomes after 36-month follow-up. Urology. 2013 Apr;81(4):781–6. https://doi.org/10.1016/j.urology.2013.01.017
- [24] Vertosick EA, Vickers AJ, Cowan JE, et al. Interpreting patient reported urinary and sexual function outcomes across multiple validated instruments. J Urol. 2017 Sep;198(3):671–7. https://doi.org/10.1016/j.juro.2017.03.121