ORIGINAL RESEARCH ARTICLE
Cystectomy for bladder cancer in Sweden – short-term outcomes after centralization
Fredrik Liedberga,b, Oskar Hagbergb, Firas Aljaberyc, Ove Andrénd, Victor Falinie, Truls Gårdmarkf, Viveka Ströckg and Tomas Jerlströmh
aDepartment of Urology Skåne University Hospital, Malmö, Sweden; bInstitution of Translational Medicine, Lund University, Malmö, Sweden; cDepartment of Clinical and Experimental Medicine, Division of Urology, Linköping, University, Linköping, Sweden; dSection of Urology, Department of Surgery, Skellefteå Hospital, Sweden; eRegional Cancer Center South, Lund, Sweden; fDepartment of Clinical Sciences, Danderyd Hospital, Karolinska Institute, Stockholm, Sweden; gDepartment of Urology, Sahlgrenska University Hospital and Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sweden; hDepartment of Urology, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
ABSTRACT
Objective: Radical cystectomy (RC) for bladder cancer is associated with an inherent risk of complications and even postoperative mortality. The number of hospitals performing RC has decreased in Sweden over time, and since a formal regional centralization in 2017 cystectomy care is currently provided by nine hospitals.
Material and methods: In the Swedish National Urinary Bladder Cancer Register (SNRUBC) 90-day complications after RC have been registered with high coverage since 2012. Descriptive data and short-term outcomes were compared in relation to centralization of the cystectomy care by stratifying data before (2012–2016) and after (2017–2023).
Results: Out of all 4,638 cystectomies, 2,738 (59%) were performed after the centralization in 2017 and onwards. The median age at RC increased from 71 (Inter Quartile Range [IQR] 65–76) to 73 (IQR 67–77) years, and the proportion of patients with comorbidity (American Society of Anesthesiologists [ASA] 3 or 4) increased from 32% to 37% after the centralization (p < 0.001). The number of patients suffering from high-grade complications within 90 days of surgery corresponding to Clavien grade three were 345 (18%) and 407 (15%), and corresponding to Clavien grade four 61 (3%) and 64 (2%) before and after centralization, respectively. Reoperations within 90 days of RC decreased from 234/1,900 (12%) to 208/2,738 (8%) (p < 0.001), and 90-day mortality decreased from 84/1,900 (4%) to 85/2,738 (3%) (p = 0.023) before and after centralization, respectively.
Conclusion: After the centralization of the cystectomy-care in Sweden, older patients and individuals with more extensive comorbidity were offered RC whereas 90-day mortality and the proportion of patients subjected to reoperations within 90 days of surgery decreased without increasing waiting times.
KEYWORDS: bladder cancer; radical cystectomy; centralization; hospital volume
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 84–89. https://doi.org/10.2340/sju.v59.40120.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 15 February 2024; Accepted: 15 April 2024; Published: 29 April 2024
CONTACT Fredrik Liedberg fredrik.liedberg@med.lu.se Department of Urology, Skåne University Hospital, Department of Translational Medicine, Lund University, Jan Waldenströms gata 7, SE-205 02 Malmö, Sweden
Competing interests and funding: None of the authors have any conflicts of interest to report.
This work was supported by the Swedish Cancer Society (grant numbers CAN 2020/0710 and CAN 2023/2807), Swedish Research Council (2021-00859), Lund Medical Faculty (ALF), The Cancer Research Fund at Malmö General Hospital, Skåne County Council’s Research and Development Foundation (REGSKANE-622351), The Hjelm Family Foundation for Medical Research, Gyllenstiernska-Krapperup Foundation, Gösta Jönsson Research Foundation, The Foundation of Urological Research (Ove and Carin Carlsson bladder cancer donation), Maud and Birger Gustavsson Research Fund, and Hillevi Fries Research Foundation. The funding sources had no role in the study design, data analyses, interpretation of the results, or writing of the manuscript.
Introduction
Neoadjuvant chemotherapy (NAC) and radical cystectomy (RC) with extended lymphadenectomy is the treatment standard for muscle-invasive bladder cancer (MIBC), and RC is also recommended for primary non-muscle invasive bladder cancer (NMIBC) with very high risk of progression. In men RC includes extirpation of the prostate, and in women the anterior vaginal wall and internal genitalia are also excised en bloc with the urinary bladder. Today, the most common urinary diversion after RC is an ileal conduit, and the use of continent diversion has declined over the years, also in countries with previously a more frequent use of continent cutaneous pouches and orthotopic bladder substitutes [1]. Such a shift in practice patterns for urinary diversion, together with improved diagnostic and interventional methods to treat complications after RC might have improved short-term outcomes after RC over time. The scientific endeavor to investigate the association between surgical volume and outcomes was initiated over four decades ago [2]. For such complex surgery as RC, higher hospital volumes have been linked to decreased postoperative mortality and quality of care [3], as well as long term survival [4]. Between 1997 and 2014, RC was performed in 44 different hospitals in Sweden [4]; and based on available evidence on the association between hospital volume and outcomes, a centralized cystectomy care commenced in January 1st 2017 [5]. Awaiting longer follow-up to also enable studying survival outcomes, we investigate indications, lead times and short-term outcomes after RC in Sweden 5 years before centralization with the subsequent 7 years up to now.
Material and methods
In the Swedish National Urinary Bladder Cancer Register (SNRUBC) complications 90 days after RC have been registered since 2011, but with high coverage since 2012, where data are presented in an interactive and open online data resource (RODRET) [6]. The aim of SNRUBC is to assess adherence to national guidelines [7], monitor quality-indicators for bladder cancer care; it has also been the basis for studying different aspects of bladder cancer management [8, 9].
From January 1st 2017, RC was performed in 10 hospitals in Sweden, which was further reduced to currently 9 units compared to 24 hospitals in 2012 [5]. Data for all patients registered with RC as primary treatment for patients with date of surgery between 2012 and 2023 were retrieved from SNRUBC in January 2024. Information about indications (proportion of NMIBC subjected to RC), patient and treatment characteristics, and complications 90 days after RC (proportion high grade complications (Clavien grade 3-5), reoperations, and mortality at 90 days) were stratified before and after centralization January 1st 2017 (i.e., 2012–2016 vs. 2017–2023). Additionally, lead times, that is time from urology referral to date of first urologic outpatient consultation, transurethral resection of the bladder tumor (TURB), and RC, were stratified by year of diagnosis comparing before and after centralization (2012–2016 vs. 2017–2023).
Only descriptive statistics applying chi-squared test to compare proportions of categoric variables and Wilcoxon rank-sum test to compare medians of continuous variables between the two groups were used. To visualize outcomes from each hospital, the proportion of positive outcomes are displayed in a funnel plot [10]. A funnel plot is created by calculating intervals so that the probability is 95% and 99%, respectively, for a hospital’s proportion to be inside the interval if positive outcome is equally probable at each hospital (hypergeometric distribution). If a hospital’s proportion is outside any of these intervals, this is a signal that the deviation is larger than could be explained by chance. The intervals are arranged from bottom to top in order of increasing hospital size, and consequently the intervals are narrower the larger the hospital is, which creates the characteristic ‘funnel’ shape. For all statistical analyses, the R statistical package version 4.2.2 was used.
Ethical approval for the study was obtained (EPM 2020-02397).
Results
Out of the 4,638 cystectomies registered in 2012–2023, 2,738 (59%) were performed after the centralization of RC in January 2017. The median age at cystectomy increased from 71 (IQR 65–76) years before 2017 to 73 (IQR 67–77) years after the centralization. The proportion of patients with comorbidity corresponding to an American Society of Anesthesiologists (ASA) score 3 or 4 also increased, from 32% before the centralization to 37% after 2017 (p < 0.001). NMIBC was the indication for RC in 680 (36%) patients operated prior to 2017, increasing to 1,101 (40%) after centralization of RC. Use of NAC in patients with MIBC increased from 515/889 (58%) to 724/1,084 (67%) (p < 0.001) after the centralization. Also, the use of robotic-assisted radical RC (RARC) increased during the whole period, from 42/272 (15%) in 2012 and is since 2021 the most common type of RC in Sweden. In 2023, 140/257 (54%) patients were operated with RARC. Lymphadenectomy to the aortic bifurcation was more frequently applied after 2017, with 383 (20%) receiving such surgery prior to 2017 compared to 730 (27%) after centralization of the cystectomy care (p < 0.001). On the other hand, patients operated prior to 2017 were twice as often subjected to continent urinary diversion (12%) (continent cutaneous pouch or orthotopic neobladder) compared to after the centralization (6%) (Table 1).
Median delay from referral to RC without NAC decreased from 110 (IQR (81–146) days to 98 (IQR 75–132) days after the centralization (p = 0.005). During the same time-periods, time from referral to urologic outpatient consultation decreased from 14 (IQR 4–26) days to 10 (IQR 4–19) days (p < 0.001), and the delay from referral to TURB decreased from 35 (IQR 20–56) days to 27 (IQR 16–41) days (p < 0.001).
Unplanned readmissions within 90 days of RC occurred in 28% of patients operated before and after the centralization. The number of patients registered with high-grade complications within 90 days of surgery corresponding to Clavien grade three were 345 (18%) and 407 (15%), and corresponding to Clavien grade four were 61 (3%) and 64 (2%) before and after centralization, respectively (p < 0.001). Mortality 90 days after RC was 19/274 (7%) in 2012, but decreased over time (Figure 1), and before centralization 85/1,900 (4%) patients died within 90 days of RC compared to 85/2,738 (3%) after the centralization (p = 0.023). Similarly, reoperations within 90 days of surgery decreased over time (Figure 2), from 234/1,900 (12%) before to 208/2,738 (8%) after centralization (p < 0.001). The distribution of proportions 90-day mortality between hospitals before and after centralization of cystectomy care in Sweden is displayed for each hospital throughout Figures 3 and 4.
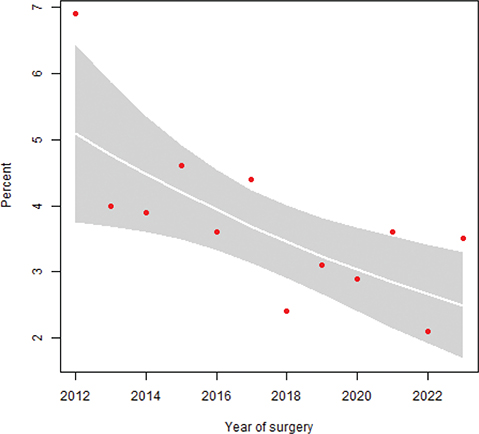
Figure 1. Proportion 90-day mortality after RC over time with 95% confidence intervals (CI) (p = 0.023).
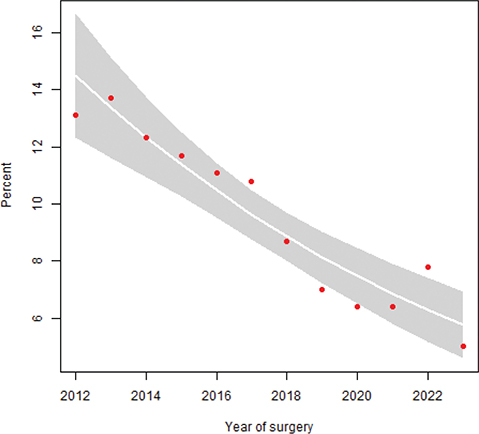
Figure 2. Proportion reoperation within 90 days of RC stratified by year of surgery with 95% CI (p < 0.001).
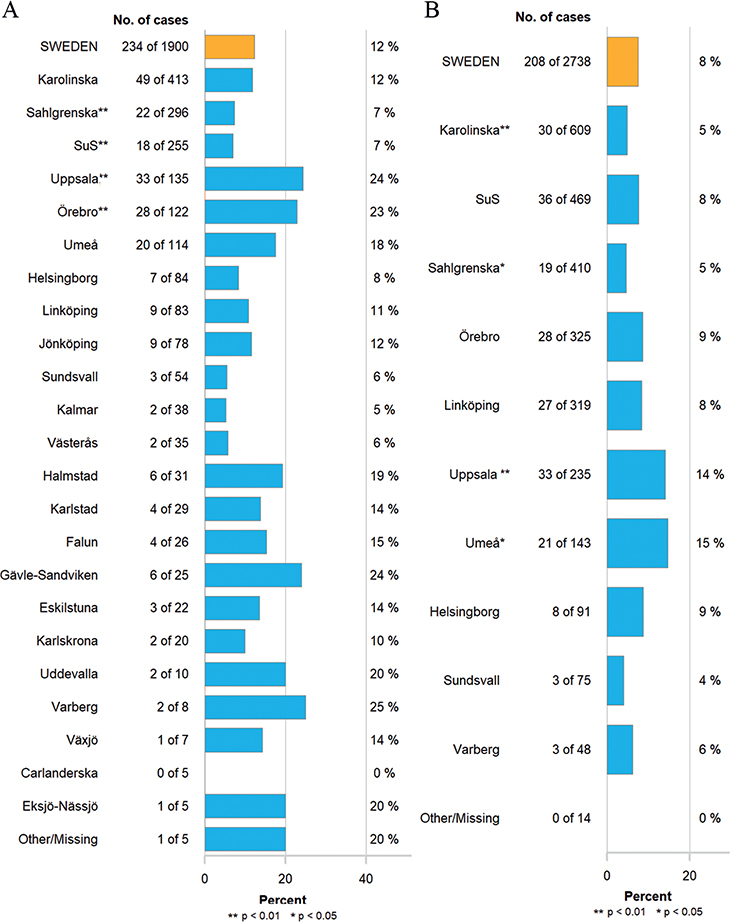
Figure 3. Proportion of reoperations within 90 days of surgery in hospitals performing RC (A) before centralization 2012–2016 and (B) after centralization 2017–2023. p-values compared to the whole country. SuS: Skåne University Hospital.
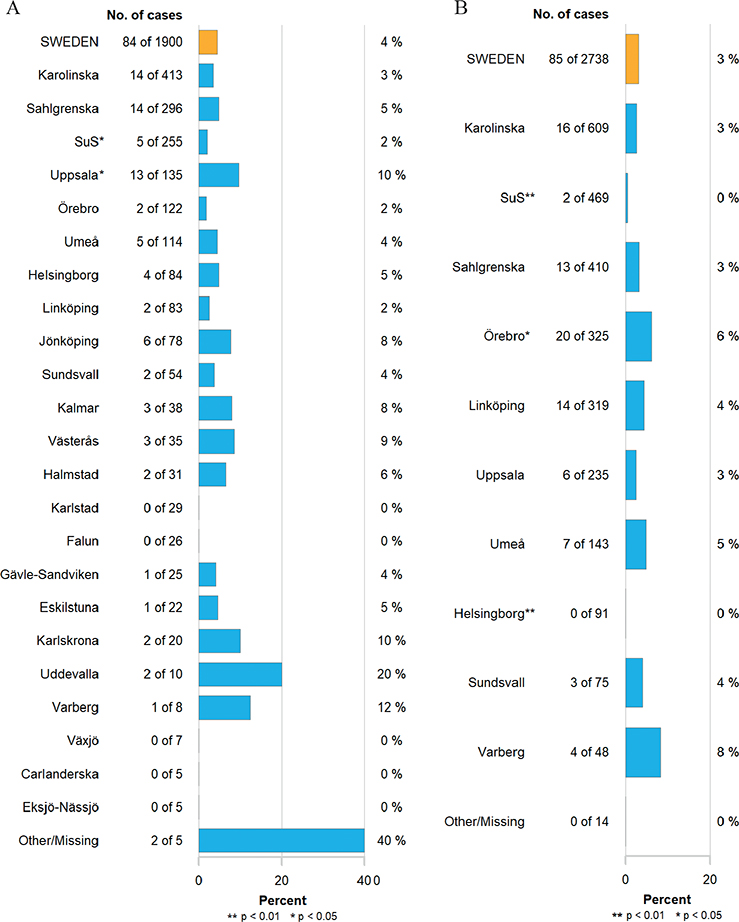
Figure 4. 90-day mortality rates per hospital (A) before centralization 2012–2016 and (B) after centralization 2017–2023. p-values compared to the whole country. SuS: Skåne University Hospital.
Discussion
Since centralization of the cystectomy care in 2017, older and more comorbid patients are subjected to RC in Sweden. At the same time, the 90-day mortality rate and proportion of patients reoperated within 90 days of RC have decreased.
The current study was undertaken in data from the SNRUBC with a high coverage for the investigated years [11], although some underreporting of high-grade complications Clavien grade 3–5 was identified at third party validation of the primary registration [12]. Both under- and overreporting of 90-day mortality has also been reported, mainly related to erroneous reporting of dates into the SNRUBC [11]. Nonetheless, the decline in 90-day mortality observed over time in the current study is more pronounced compared to the postoperative mortality reduction reported after centralization of cystectomy care in England, where 90-day mortality decreased from 10% in 1998 to 5% in 2012 [13]. The lowest national 90-day mortality rate has been published from Iceland (1/108 [1%]), where one surgeon performed the vast majority of all procedures [14], although surgeon volume is likely less important than hospital volume regarding association with postoperative morbidity and mortality. Still, one limitation in the current study is the lack of data on surgeon volume, and consequently no formal mediation analysis disentangling the individual role of surgeon and hospital volume on the outcomes after centralization of RC in the current study were possible. Considering the gradually declining proportion of patients subjected to a reoperation within 90 days of RC, from 13% in 2012 to 5% in 2023, the current reoperation rate in Sweden is lower compared to 12% reported in a recently published systematic review [15].
It is not possible to claim causality between centralization of cystectomy care and the improved short-term outcomes identified in the current study, although other studies have reported similar improvements when associating hospital volume RC and 90-day mortality [4, 16, 17]. Other parallel developments over time such as the introduction of restrictive perioperative fluid administration [18], implementation of the early recovery after surgery (ERAS) concept [19], and the decreased use of continent reconstruction [20], might also have contributed to the observed decrease in 90-day mortality and reoperation rates over time. Based on randomized data, the risk of complications after RC increases when performed in older individuals with comorbidity such as hypertension, diabetes, cardiovascular and pulmonary diseases [21]. Nonetheless, based on an increased median age at RC after centralization in the current study (73 vs. 71 years prior to 2017) and an increased proportion of patients subjected to RC with American Society of Anesthesiologists (ASA) score 3 or 4 (37% after centralization compared to 32% prior to 2017), it is unlikely that patients more selectively are offered RC after centralizing the cystectomy care in Sweden.
Prior to the centralization of RC in Sweden, concerns were raised that an increased workload in fewer units performing RC would increase the delay to RC. As standardized care pathways for bladder cancer were introduced in September 2015, it is not possible to disentangle if the centralization per se affected lead times. Thus in 2015, national lead time goals were for the first time defined in conjunction with investigating macroscopic hematuria, including diagnostic procedures and time to RC. Since this health care reform was launched, time from referral to RC decreased from 110 to 98 days in 2023, which still is too long based on reported associations between treatment delay in conjunction with RC [22]. On the other hand, the previously reported worsened survival outcomes after bladder cancer surgery during holiday periods in Sweden have not been confirmed for RC until 2014, when both similar disease-specific and overall survival were similar during the whole year [23].
Another change in cystectomy care in Sweden occurring parallel to centralization was the dissemination of RARC, and since 2021 RARC is more commonly performed than open RC. Between 2011 and 2019, patients operated with RARC were more frequently subjected to unscheduled readmissions within 90 days of surgery (29% vs. 25% after open RC), whereas high-grade complications (Clavien grade 3–5) and reoperations within 90 days were less common after RARC odds ratio (OR) 0.58 (95% CI 0.47–0.72) and OR 0.53 (95% CI 0.39–0.71), respectively) compared to open RC [24]. Thus, the simultaneous transition to RARC during centralization of RC might also have affected short-term outcomes in the current study. A recently published cost effective-analysis comparing the two surgical methods found that RARC is more expensive [25], although the presence of surgical complications is to a larger extent than the surgical technique (RARC or open RC) determining the variation in costs after RC [26]. An additional factor contributing to improved short-term outcomes after RC in Sweden during the study period might be the registration of 90-day complications in the SNRUBC per se, that is, improved performance due to subjects’ knowledge of being observed (Hawthorne effect) [27].
The centralization of the cystectomy care in Sweden is accompanied by possibilities and needs to perform education, clinical development, and research. Currently, several prospective multicenter studies are ongoing, where patients are offered inclusion in regions within the informal network of the centralized care now treating these patients (ISRCTN1817210; ISRCTN99427820; ISRCTN64538003). Although it is likely that many other factors have contributed to improved short-term outcomes after RC reported here, the absence of increased waiting times for RC support a continued centralization of RC. This is also in line with the recent decision to nationally centralize pelvic exenterations in Sweden to three units from June 2024.
Conclusions
Since centralization of the cystectomy care in Sweden 2017, older patients and individuals with more extensive comorbidity are offered RC compared to previously. At the same time, 90-day mortality and the proportion of patients subjected to reoperations within 90 days of surgery decreased without increasing waiting times, justifying the centralization of RC in Sweden.
References
- [1] Klemm J, Fisch M, Laukhtina E, et al. Continent diversion is losing its momentum: a nationwide trend analysis from Germany 2005–2021. BJU Int. 2023. https://doi.org/10.1111/bju.16215
- [2] Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. https://doi.org/10.1056/NEJM197912203012503
- [3] Bruins HM, Veskimäe E, Hernández V, et al. The importance of hospital and surgeon volume as major determinants of morbidity and mortality after radical cystectomy for bladder cancer: a systematic review and recommendations by the European Association of Urology muscle-invasive and metastatic bladder cancer guideline panel. Eur Urol Oncol. 2020;3:131–144. https://doi.org/10.1016/j.euo.2019.11.005
- [4] Liedberg F, Hagberg O, Aljabery F, et al. Period-specific mean annual hospital volume of radical cystectomy is associated with outcome and perioperative quality of care: a nationwide population-based study. BJU Int. 2019;124:449–456. https://doi.org/10.1111/bju.14767
- [5] https://cancercentrum.se/globalassets/vara-uppdrag/nivastrukturering/remisser-och-beslutsunderlag/omgang-3/beslutsrekommendation_till_landsting-regioner_161212.pdf
- [6] https://statistik.incanet.se/Urinblasecancer/
- [7] Liedberg F, Kjellström S, Lind AK, et al. Swedish National Guidelines on Urothelial Carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand J Urol. 2022;56:137–146. https://doi.org/10.1080/21681805.2022.2041086
- [8] Abuhasanein S, Jahnson S, Aljabery F, et al. Standardized care pathways for patients with suspected urinary bladder cancer: the Swedish experience. Scand J Urol. 2022;56:227-232. https://doi.org/10.1080/21681805.2022.2058605
- [9] Lind AK, Liedberg F, Aljabery F, et al. Health-related quality of life prior to and 1 year after radical cystectomy evaluated with FACT-G and FACT-VCI questionnaires. Scand J Urol. 2023;58:76–83. https://doi.org/10.2340/sju.v58.11952
- [10] Anell A, Hagberg O, Liedberg F, et al. A randomized comparison between league tables and funnel plots to inform health care decision-making. Int J Qual Health Care. 2016;28:816–823. https://doi.org/10.1093/intqhc/mzw125
- [11] Häggström C, Hagberg O, Gårdmark T, et al. Cohort profile: Bladder Cancer Data Base Sweden (BladderBaSe) 2.0. BMJ Open. 2022;12:e064898. https://doi.org/10.1136/bmjopen-2022-064898
- [12] Böös M, Jerlström T, Beckman E, et al. Who should record surgical complications? Results from a third-party assessment of complications after radical cystectomy. Scand J Urol. 2019;53:339–343. https://doi.org/10.1080/21681805.2019.1643912
- [13] Hounsome LS, Verne J, McGrath JS, et al. Trends in operative caseload and mortality rates after radical cystectomy for bladder cancer in England for 1998–2010. Eur Urol. 2015;67:1056–1062. https://doi.org/10.1016/j.eururo.2014.12.002
- [14] Björnsson O, Gudmundsson EO, Marteinsson VT, et al. Radical cystectomy in the treatment of bladder cancer in Iceland: a population-based study. Scand J Urol. 2016;50:65–70. https://doi.org/10.3109/21681805.2015.1085088
- [15] Maibom SL, Joensen UN, Poulsen AM, et al. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open. 2021;11:e043266. https://doi.org/10.1136/bmjopen-2020-043266
- [16] Richters A, Ripping TM, Kiemeney LA, et al. Hospital volume is associated with postoperative mortality after radical cystectomy for treatment of bladder cancer. BJU Int. 2021;128:511–518. https://doi.org/10.1111/bju.15334
- [17] Motlagh SR, Mori K, Aydh A, et al. Impact of hospital and surgeon volumes on short-term and long-term outcomes of radical cystectomy. Curr Opin Urol. 2020;30:701–710. https://doi.org/10.1097/MOU.0000000000000805
- [18] Wuethrich PY, Burkhard FC, Thalmann GN, et al. Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: a randomized clinical trial. Anesthesiology. 2014;120:365–377. https://doi.org/10.1097/ALN.0b013e3182a44440
- [19] Zhou Y, Li R, Liu Z, et al. The effect of the enhanced recovery after surgery program on radical cystectomy: a meta-analysis and systematic review. Front Surg. 2023;10:1101098. https://doi.org/10.1097/ALN.0b013e3182a44440
- [20] Vrang ML, Østergren PB, Fode MM, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion: a Danish 11-year series. BJU Int. 2023;132:428–434. https://doi.org/10.1111/bju.16098
- [21] Katsimperis S, Tzelves L, Tandogdu Z, et al. Complications after radical cystectomy: a systematic review and meta-analysis of randomized controlled trials with a meta-regression analysis. Eur Urol Focus. 2023;9(6):920–929. https://doi.org/10.1016/j.euf.2023.05.002
- [22] Russell B, Liedberg F, Khan MS, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. 2020;3:239–249. https://doi.org/10.1016/j.euo.2019.09.008
- [23] Liedberg F, Hagberg O, Aljabery F, et al. Survival after radical cystectomy during holiday periods. Scand J Urol. 2021;55:276–280. https://doi.org/10.1080/21681805.2021.1938665
- [24] Bergengren O, Belozerov A, Bill-Axelson A, et al. Short term outcomes after robot assisted and open cystectomy – a nation-wide population-based study. Eur J Surg Oncol. 2023;49:868–874. https://doi.org/10.1016/j.ejso.2023.01.023
- [25] Dixon S, Hill H, Flight L, et al. Cost-effectiveness of robot-assisted radical cystectomy vs open radical cystectomy for patients with bladder cancer. JAMA Netw Open. 2023;6:e2317255. https://doi.org/10.1001/jamanetworkopen.2023.17255
- [26] Leow JJ, Cole AP, Seisen T, et al. Variations in the costs of radical cystectomy for bladder cancer in the USA. Eur Urol. 2018;73:374–382. https://doi.org/10.1016/j.eururo.2017.07.016
- [27] Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. https://doi.org/10.1056/NEJMsa0810119