ORIGINAL RESEARCH ARTICLE
End-stage renal disease after renal cancer surgery: risk factors and overall survival
John Åkerlunda,b* , Börje Ljungbergd, Sven Lundstama,b,c, Ralph Peekera,b, Erik Holmbergc, Marianne Månssona and Anna Grenabo Bergdahla,b
, Börje Ljungbergd, Sven Lundstama,b,c, Ralph Peekera,b, Erik Holmbergc, Marianne Månssona and Anna Grenabo Bergdahla,b
aDepartment of Urology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; cDepartment of Urology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden; bDepartment of Oncology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; dDepartment of Surgical and Perioperative Sciences, Umeå University, Umeå, Sweden
ABSTRACT
Objective: Several risk factors for end-stage renal disease (ESRD), in patients undergoing surgical treatment for renal cell carcinoma (RCC), have been suggested by others. This study aimed to investigate such risk factors and disclose the effect of developing ESRD, postoperatively, on overall survival. The risk of developing ESRD after RCC diagnosis was also evaluated.
Material and methods: The data of 16,220 patients with RCC and 162,199 controls were extracted from the Renal Cell Cancer Database Sweden, with linkages across multiple national registers between 2005 and 2020. Cox proportional hazards regression, Kaplan–Meier curves and cumulative incidence were used for statistical analysis.
Results: The 5-year cumulative incidence of ESRD following RCC diagnosis was 2.4% (95% confidence interval [CI] 2.1–2.6) and 0.4% (95% CI 0.3–0.4) for the patients with RCC and controls, respectively. Age, chronic kidney disease, higher T-stage and radical nephrectomy (RN) were significant risk factors for ESRD within 1-year of surgery. A total of 104 and 12,152 patients with and without ESRD, respectively, survived 1-year postoperatively. The 5-year overall survival rates of patients with ESRD and those with RCC only were 50% (95% CI 0.40–0.60) and 80% (95% CI 0.80–0.81), respectively.
Conclusions: Patients who developed ESRD following renal cancer surgery had significantly poorer survival outcomes. Advanced age, comorbidities, higher-stage tumours and RN were identified as risk factors for developing ESRD. Surgical decisions are crucial. Efforts to spare renal function, including nephron-sparing surgery and active surveillance in appropriate cases, are highly relevant to reduce the development of severe kidney dysfunction.
KEYWORDS: Renal cell carcinoma; treatment; end-stage renal disease; mortality
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 109–116. https://doi.org/10.2340/sju.v59.40322.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 8 March 2024; Accepted: 24 April 2024; Published: 15 May 2024
CONTACT John Åkerlund john.akerlund@me.com Department of Urology, Institution Clinical Sciences, Sahlgrenska Academy, University of Gothenburg and Sahlgrenska University Hospital, Bruna stråket 11, SE-405 83 Gothenburg, Sweden
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v59.40322
Competing interests and funding: The authors report no conflict of interest.
Introduction
Surgical intervention remains the treatment of choice for localised renal cell carcinoma (RCC). Although surgical intervention is associated with good oncological outcomes, the subsequent nephron loss increases the susceptibility of patients to chronic renal failure and its complications. Patients with RCC and chronic kidney disease (CKD) are at an increased risk of developing end-stage renal disease (ESRD) owing to advanced age, history of diabetes or hypertension and smoking, which are predisposing factors for both conditions [1–3]. Recent studies investigating the risk of developing ESRD after RCC treatment [4–6] have revealed that approximately 2% of patients who had a normal renal function before undergoing surgery for renal cancer develop ESRD within 10 years of follow-up [6]. ESRD is a life-threatening condition, and the 5-year adjusted overall survival (OS) rate of patients undergoing haemodialysis ranges from 35 to 45 [7].
Partial nephrectomy (PN) facilitates the preservation of renal function and is consequently the preferred approach for patients with clinically localised renal masses, when feasible [8–10]. The technical limits are extended to resect more complex tumours at higher stages to conserve nephrons and possibly enhance OS in patients with larger tumours [11, 12]. Few studies have evaluated the short- and long-term postoperative renal function and overall mortality following surgical treatment for RCC [12–14]. Therefore, first (as a background), we aimed to compare cumulative incidence of ESRD in subjects with and without RCC. Second, we aimed to assess risk factors for ESRD in patients who underwent surgery for RCC. Third, we aimed to compare survival in those who developed ESRD and those who did not, during the first year after surgery for RCC.
Material and methods
Data
The Renal Cell Cancer Database Sweden (RCCBaSe), a Swedish multi-register that included the National Swedish Kidney Cancer Register (NSKCR), Swedish Renal Registry, National Patient Register and Cause of Death Register, was used to identify cases and controls. All patients enrolled in NSKCR who had been diagnosed with RCC between 2005 and 2020 and were followed-up until the date of death, emigration, or 31st December 2022, were included.
Ten unique controls matched for sex, age and county of residence were selected from the Population Register comprising all Swedish citizens for each patient with RCC selected from the RCCBaSE, as described by Landberg et al. [15]. The controls were free of RCC at the end of the year of diagnosis of the index case. Patients and controls who developed ESRD were identified via the Swedish Renal Registry and the National Patient Register. Patients with stage 5 CKD and those who had undergone renal transplantation or were undergoing dialysis after RCC diagnosis were considered to have developed ESRD [3]. The covariates for RCC diagnosis included age, sex, obesity, diabetes, hypertension, CKD stages II–IV and Tumour, Node, Metastasis stage. Additionally, data on histopathological findings, tumour grade, pT stage and type of treatment postoperatively were also extracted.
The study cohort was categorised into three groups for analysis as outlined in the introduction (Figure 1). Group I consisted of RCC patients and controls free of ESRD, with analysis focusing on the risk of ESRD after RCC diagnosis. Group II included RCC patients after surgery, examining ESRD risk factors. Group III encompassed RCC survivors 1 year after surgery, analysing survival rates with respect to ESRD development. This study was approved by the Regional Ethics Committee of Northern Sweden (diary number [Dnr]: 2012-418-31M, Dnr: 2014-301-32M, Dnr: 2015-202-32M, 2019-2579 and 2020-5093).
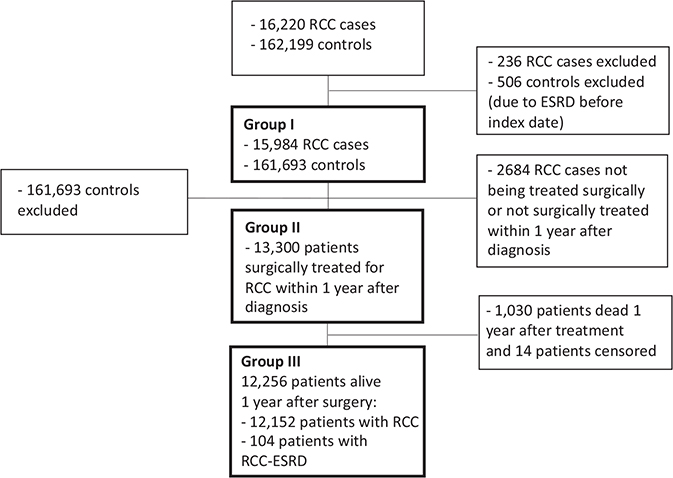
Figure 1. Flow diagram of the study selection process, 2005–2022.
RCC: Renal cell carcinoma; ESRD: End-stage renal disease.
Statistics
In analysis I, a competing risks approach was used to determine the cumulative incidence of ESRD to estimate the absolute risk of ESRD after the date of RCC diagnosis. The date of RCC diagnosis was considered the date of commencing follow-up for the patients with RCC and matched controls. The patients were followed-up until ESRD incidence, death, emigration, or end of follow-up (whichever occurred first). Death was set as a competing event, and the remaining endpoints were set as censuring events. Cause-specific univariable and multivariable Cox proportional hazards regressions were performed to calculate the hazards ratios (HRs) for a 10-year follow-up period to reflect the biological effects between patients with RCC and controls. Furthermore, a subgroup analysis was performed in order to investigate if the existence of CKD was the only driver behind the development of ESRD (post hoc). In analysis II, a Cox proportional hazards regression was performed to calculate the HRs for risk factors associated with end-stage renal disease in patients with RCC after treatment based on different patient, tumour and treatment characteristics. In analysis III, Kaplan–Meier estimates and Cox proportional hazards regression were used to analyse the OS. To compare the survival of patients with RCC with and without prior ESRD, they were divided into two groups: those who developed ESRD and those who did not develop ESRD in the first year after treatment and were alive at 1 year.
In all Cox regressions, the assumption of proportional hazards was tested using Schoenfeld’s residuals. Due to non-proportionality, the HRs are reported separately for 0–1 year and 1–10 years in analysis I and II, respectively. Statistical significance was set at P < 0.05. All statistical analyses were performed using Stata version 18.0 for Mac (StataCorp. 2023, College Station, TX, StataCorp LLC). The Fine and Grey models were fitted using the Stata macro program to estimate the cumulative incidence [16].
Results
Patients
The study population comprised 16,220 patients with RCC and 162,199 matched controls. Tables 1 and 2 present the baseline characteristics of the study population. The median follow-up was 7.3 years (interquartile range 4.4–11.2 years) for the cases and controls. Group I comprised 15,984 patients with RCC and 161,693 controls who did not have ESRD before the index date. Group II comprised 13,300 patients who underwent surgical treatment for RCC. Group III comprised 12,256 patients with RCC who survived 1 year postoperatively.
Risk of developing ESRD after RCC diagnosis
Amongst the 15,984 patients with RCC, 558 developed ESRD during the follow-up period after RCC diagnosis. In contrast, only 1,081 of the 161,693 controls developed ESRD. The prevalence of comorbidities was higher in patients with RCC than in the controls. Hypertension, diabetes, CKD and obesity were the most common comorbidities (Table 1). Amongst patients with RCC, the 5- and 10-year cumulative incidences of ESRD following RCC diagnosis were 2.4% (95% confidence interval [CI] 2.1–2.6) and 3.8% (95% CI 3.5–4.2), respectively. The corresponding risks amongst controls without RCC were 0.4% (95% CI 0.3–0.4) and 0.7% (95% CI 0.6–0.7), respectively (Figure 2). Death as a competing event is presented in Supplementary Figure 1, which depicts the cumulative incidence of death in cases and controls. Comparison between the patients with RCC and controls revealed that the HRs for developing ESRD were 17.3 (95% CI 13.5–22.3, P < 0.001) and 6.01 (95% CI 5.30–6.82, P < 0.001) for the first year and years 1–10, respectively. Comparing the risk of developing ESRD amongst patients with RCC and controls with pre-existing CKD at the index date revealed that patients with RCC had a 74% higher risk of developing ESRD (HR 1.74, 95% CI 1.40–2.16; P < 0.001), after adjusting for age and sex.
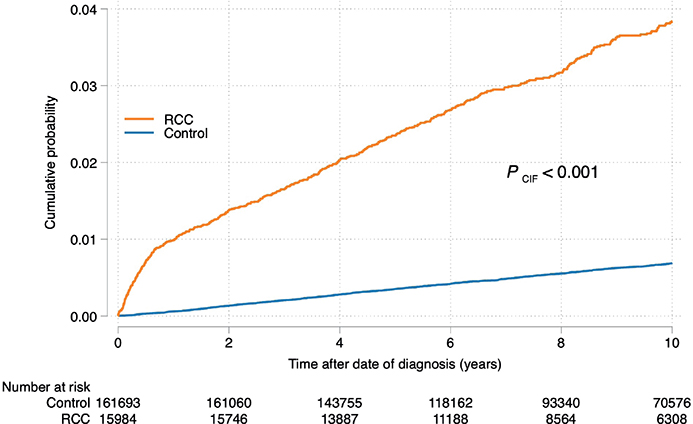
Figure 2. Cumulative incidence of the development of end-stage renal disease in the renal cell carcinoma cases and controls.
Risk factors for ESRD after RCC surgery
Overall, 13,300 patients with RCC underwent radical nephrectomy (RN) (9,077, 68%), PN (3,528, 27%) or thermal ablation (TA; 695, 5%) (Table 2). A total of 436 (3.3%) patients, including 317 (3.5%) who had undergone RN, 89 (2.5%) who had undergone PN and 30 (4.3%) patients who had undergone TA, developed ESRD within 10 years of surgery. Table 3 presents the results of the univariable and multivariable analyses of the potential risk factors for developing ESRD postoperatively over a 10-year period, segmented into 0–1 and 1–10 years postoperatively. Multivariable analysis for the first year revealed that age >60 years, diabetes, CKD, T-stage and RN were significant risk factors for ESRD development postoperatively. Age >70 years, male sex, hypertension, diabetes, CKD and T-stage were significant risk factors for ESRD development 1–10 years postoperatively. Pre-existing CKD (n = 104) was the most significant risk factor for developing ESRD after treatment across both follow-up periods, with HRs of 22.5 (95% CI 12.8–39.5; P < 0.001) and 9.64 (95% CI 6–15.5; P < 0.001) for 0–1 and 1–10 years, respectively.
Survival
The 5- and 10-year cumulative incidences of OS were 74% (95% CI 0.73–0.75) and 56% (95% CI 0.55–0.57), respectively, amongst the 13,300 patients who had undergone treatment for RCC (Supplementary Figure 2). A total of 129 patients were diagnosed with ESRD (RCC-ESRD) within the first year of renal cancer treatment. Amongst them, 25 (19%) were deceased by the 1-year mark following treatment (RN, 23 patients; PN, one patient; and TA, one patient). In contrast, only 1,005 (8%) out of 12,256 patients with RCC who did not develop ESRD (RCC only) were deceased. The remaining 104 patients with RCC-ESRD and 12,152 patients with RCC who survived at year 1 were followed-up to determine the OS. The 5-year OS rates of the patients with RCC-ESRD and RCC only were 50% (95% CI 0.40–0.60) and 80% (95% CI 0.80–0.81), respectively (Figure 3). Amongst the 307 patients with RCC only who developed ESRD during the follow-up period, 157 died within the 10-year follow-up period. Comparison of the patients with RCC with those with RCC-ESRD revealed that the HR for death beginning at 1 year postoperatively was 3.06 (95% CI 2.41–3.89, P < 0.001) in the univariable analysis. Multivariable analysis yielded an HR of 2.00 (95% CI 1.56–2.57, P < 0.001) after adjusting for age, sex, obesity, diabetes, hypertension, CKD, T-stage and treatment type (RN, PN and TA).
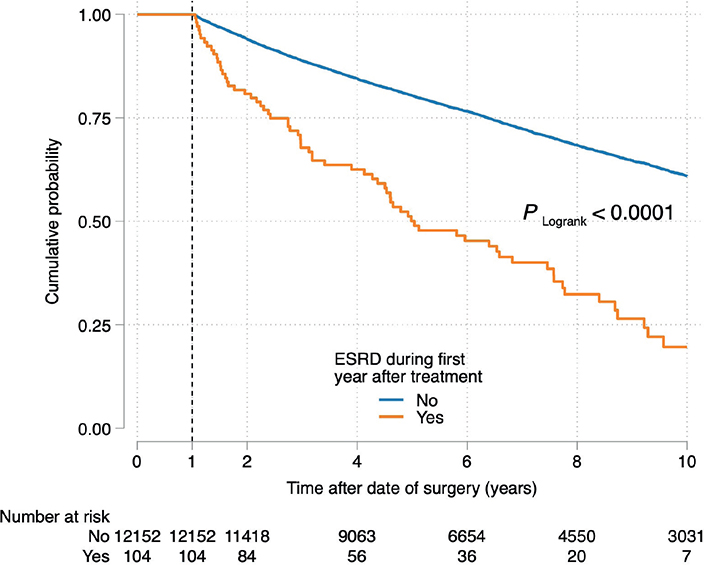
Figure 3. Overall survival probability of the patients with renal cell carcinoma who developed end-stage renal disease and patients with renal cell carcinoma only 1 year after treatment.
Discussion
The present study revealed that 3.3% of patients surgically treated for RCC developed ESRD within 10 years postoperatively. Older age, male sex, higher T-stage, hypertension, diabetes, preoperative CKD and RN were identified as significant risk factors for ESRD development following RCC surgery. Amongst the patients who survived 1 year postoperatively, the 5-year OS rates of patients with RCC-ESRD and those with RCC only were 50% and 80%, respectively.
The risk of developing ESRD within 5 years after being diagnosed with RCC was sevenfold higher in affected individuals than in controls, corroborating previous reports [2, 6, 17]. A history of CKD before RCC diagnosis is a strong predictor of developing ESRD after RCC diagnosis [2, 18]. When comparing patients with RCC and controls with pre-existing CKD in this study, patients with RCC had a 74% higher risk of developing ESRD than the controls did. Hence, pre-existing CKD in patients with RCC does not completely account for the increased risk of developing ESRD observed in this group.
Thus, the risk of developing ESRD after RCC diagnosis is high, particularly within the first year. Several significant risk factors for ESRD development after RCC surgery were identified in the present study. The most significant factors were CKD, age >60 years, T-stage and RN (0–1 year postoperatively). The treatment type became insignificant within 1–10 years postoperatively. In contrast, diabetes, hypertension and age >70 years were the most important risk factors after 1 year. Compared with RN, PN was associated with a 55% risk reduction within 1 year. This finding, along with those of previous studies [17, 19–21], underscores the importance of renal preservation in decreasing ESRD risk and improving OS. However, patients undergoing RN generally present with larger tumours and more comorbidities than those undergoing PN do, indicating that patients who may benefit the most from nephron-sparing surgery are not usually eligible for this treatment [22]. This may explain the poorer OS of the patients who undergo RN and illustrates the challenge of selecting appropriate treatments or active surveillance for different patients [23, 24].
Patients without medical risk factors generally achieve a more favourable survival prognosis after RN, even when diagnosed with CKD postoperatively [2, 25, 26]. Previous studies have shown that CKD does not generally progress in patients with surgically induced CKD [13, 27], whereas patients with prevalent factors such as CKD, diabetes and cardiovascular disease have a substantially higher risk of progression to a more severe CKD-stage or even ESRD, owing to the combination of medically and surgically induced CKD [13, 25, 27, 28]. Approximately 14% of patients diagnosed with CKD underwent surgical intervention in this study, indicating that a selection bias was likely, whereby patients with medically induced CKD prior to RCC diagnosis were deemed ineligible for surgery owing to the heightened risk of progression to ESRD. Moreover, 36% (data not shown) of the patients with CKD who received treatment developed ESRD during the follow-up period. However, only 0.8% had CKD at the time of surgery, suggesting that although this condition represents a significant risk factor, it pertains to a relatively small fraction of the patient population.
A clear decline in OS was observed in patients who developed ESRD following treatment compared with that in those with RCC only, in the present study. Patients with RCC-ESRD had significantly poorer survival rates even after adjusting for the underlying causes of ESRD. This analysis further supports the previously documented poor prognosis amongst patients with RCC-ESRD [18, 25, 29].
Renal function and overall survival may be better in patients with surgically induced CKD than in those with medical causes [29]. Furthermore, patients with surgically induced CKD have a lower risk of non-cancer mortality and a greater chance of being eligible for renal transplantation than those with medically induced ESRD do [13]. Survival outcomes of patients with surgically induced CKD resemble those of patients without CKD more closely than those of patients with combined medical-surgical CKD [27]. Lane et al. reported that non-cancer mortality rates at 5 years were 6%, 9% and 20% in patients without CKD, those with surgical CKD and patients with medical-surgical CKD, respectively [25].
Patients diagnosed with renal cancer require a treatment option that yields the best prospects for survival and preserves quality of life whilst minimising the risk of kidney dysfunction necessitating dialysis. The data herein underscore the importance to consider age, kidney function and comorbidities before commencing treatment.
The present study has some limitations. First, the register-based cohort approach faces inherent limitations as the data were collected from various hospitals. This precluded complete uniformity in data collection and resulted in potential coding discrepancies. Although the follow-up duration was substantial, survival outcomes may change over time owing to advancements in medical and surgical management. Notably, treatments, such as nephron-sparing surgery, robot-assisted surgery, active surveillance and the increasingly widespread adoption of ablative techniques, were less accessible at the beginning of the study period. Thus, our results highlight risk factors from the past treatment era of 2005–2020, which does not encompass the full range of treatment modalities available today or for the patients in the near future. This is a limitation because it might restrict the use of our findings in upcoming treatment settings. Nonetheless, the significance of this research area is evident, and it warrants further investigation and assessment in the years to come. Finally, the data in this study rely on Swedish registries, which may have unique referral and treatment patterns not applicable to other countries.
We have acknowledged that whilst we have attempted to adjust for known confounders and risk factors, the potential for residual confounding remains, as is the case in most observational studies.
Nevertheless, the current study has several strengths, including a nationwide population base, an extensive follow-up period and comprehensive coverage of the entire population, encompassing 99% of all cases of RCC in Sweden. Moreover, the NSKCR has demonstrated high comparability and validity in a cohort study using registry data [30]. Furthermore, RCCBaSe includes several high-quality population-based registers encompassing the same national population. The present study is the largest population-based study to assess the risk factors for ESRD following RCC treatment and compare the OS between patients with RCC-ESRD and those with RCC only.
Conclusions
Following surgery for RCC, patients with comorbidities, higher-stage tumours and those undergoing RN are at greater risk of developing ESRD. Furthermore, patients who develop ESRD after renal cancer surgery have significantly poorer survival. Efforts to spare renal function, including nephron-sparing surgery and active surveillance in appropriate cases, are highly relevant to reduce the development of severe kidney dysfunction.
Acknowledgements
Thanks to the members of the National Swedish Kidney Cancer Register steering committee and collaborators at the Regional Cancer Centre, Stockholm, for providing data from the register. This work was supported by funds from Märta and Gustaf Ågren’s research foundation.
References
- [1] Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 2010;106(4):489–92. https://doi.org/10.1111/j.1464-410X.2009.09147.x
- [2] Hung PH, Tsai HB, Hung KY, Muo CH, Chung MC, Chang CH, et al. Increased risk of end-stage renal disease in patients with renal cell carcinoma: a 12-year nationwide follow-up study. Medicine (Baltimore). 2014;93(8):e52. https://doi.org/10.1097/MD.0000000000000052
- [3] Åkerlund J, Holmberg E, Lindblad P, Stendahl M, Ljungberg B, Thorstenson A, et al. Increased risk for renal cell carcinoma in end stage renal disease – a population-based case-control study. Scand J Urol. 2021;55(3):209–14. https://doi.org/10.1080/21681805.2021.1900387
- [4] Hu SL. The Nephrologist’s management of renal cell carcinoma after kidney surgery. Semin Nephrol. 2020;40(1):59–68. https://doi.org/10.1016/j.semnephrol.2019.12.007
- [5] Ellis RJ, Edey DP, Del Vecchio SJ, McStea M, Campbell SB, Hawley CM, et al. End-stage kidney disease following surgical management of kidney cancer. Clin J Am Soc Nephrol. 2018;13(11):1641–8. https://doi.org/10.2215/CJN.06560518
- [6] Capitanio U, Larcher A, Terrone C, Antonelli A, Volpe A, Fiori C, et al. End-stage renal disease after renal surgery in patients with normal preoperative kidney function: balancing surgical strategy and individual disorders at baseline. Eur Urol. 2016;70(4):558–61. https://doi.org/10.1016/j.eururo.2016.03.023
- [7] Kramer A, Boenink R, Stel VS, Santiuste de Pablos C, Tomović F, Golan E, et al. The ERA-EDTA registry annual report 2018: a summary. Clin Kidney J. 2021;14(1):107–23. https://doi.org/10.1093/ckj/sfaa271
- [8] Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J Urol. 2021;206(2):199–208. https://doi.org/10.1097/JU.0000000000001911
- [9] Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. https://doi.org/10.1016/j.eururo.2022.03.006
- [10] Tan WS, Koelker M, Campain N, Cole AP, Labban M, Mossanen M, et al. Comparison of long-term outcomes for young and healthy patients with cT1a and cT3a renal cell carcinoma treated with partial nephrectomy. Eur Urol Focus. 2023;9(2):333–5. https://doi.org/10.1016/j.euf.2022.09.018
- [11] Kim SP, Campbell SC, Gill I, Lane BR, Van Poppel H, Smaldone MC, et al. Collaborative review of risk benefit trade-offs between partial and radical nephrectomy in the management of anatomically complex renal masses. Eur Urol. 2017;72(1):64–75. https://doi.org/10.1016/j.eururo.2016.11.038
- [12] Palacios DA, Zabor EC, Munoz-Lopez C, Roversi G, Mahmood F, Abramczyk E, et al. Does reduced renal function predispose to cancer-specific mortality from renal cell carcinoma? Eur Urol. 2021;79(6):774–80. https://doi.org/10.1016/j.eururo.2021.02.035
- [13] Bhindi B, Lohse CM, Schulte PJ, Mason RJ, Cheville JC, Boorjian SA, et al. Predicting renal function outcomes after partial and radical nephrectomy. Eur Urol. 2019;75(5):766–72. https://doi.org/10.1016/j.eururo.2018.11.021
- [14] Mason R, Kapoor A, Liu Z, Saarela O, Tanguay S, Jewett M, et al. . The natural history of renal function after surgical management of renal cell carcinoma: results from the Canadian Kidney Cancer Information System. Urol Oncol. 2016;34(11):486.e1–.e7. https://doi.org/10.1016/j.urolonc.2016.05.025
- [15] Landberg A, Lindblad P, Harmenberg U, Lundstam S, Ljungberg B, Thorstenson A, et al. The renal cell cancer database Sweden (RCCBaSe) – a new register-based resource for renal cell carcinoma research. Scand J Urol. 2020;54(3):235–40. https://doi.org/10.1080/21681805.2020.1766561
- [16] Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67(1):39–49. https://doi.org/10.1111/j.1541-0420.2010.01420.x
- [17] Lin WY, Liang FW, Lu TH. Risk of end-stage renal disease after cancer nephrectomy in Taiwan: a nationwide population-based study. PLoS One. 2015;10(5):e0126965. https://doi.org/10.1371/journal.pone.0126965
- [18] Nguyen KA, Vourganti S, Syed JS, Luciano R, Campbell SC, Shuch B. End-stage renal disease secondary to renal malignancy: epidemiologic trends and survival outcomes. Urol Oncol. 2017;35(8):529.e1–.e7. https://doi.org/10.1016/j.urolonc.2017.03.003
- [19] Sun M, Bianchi M, Hansen J, Trinh QD, Abdollah F, Tian Z, et al. Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol. 2012;62(4):696–703. https://doi.org/10.1016/j.eururo.2012.03.051
- [20] Antonelli A, Minervini A, Sandri M, Bertini R, Bertolo R, Carini M, et al. Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer-specific mortality in renal cell carcinoma. Eur Urol. 2018;74(5):661–7. https://doi.org/10.1016/j.eururo.2018.07.029
- [21] Mari A, Tellini R, Antonelli A, Porpiglia F, Schiavina R, Amparore D, et al. A nomogram for the prediction of intermediate significant renal function loss after robot-assisted partial nephrectomy for localized renal tumors: a prospective multicenter observational study (RECORd2 Project). Eur Urol Focus. 2022;8(4):980–7. https://doi.org/10.1016/j.euf.2021.09.012
- [22] Schmid M, Abd-El-Barr AE, Gandaglia G, Sood A, Olugbade K, Jr., Ruhotina N, et al. Predictors of 30-day acute kidney injury following radical and partial nephrectomy for renal cell carcinoma. Urol Oncol. 2014;32(8):1259–66. https://doi.org/10.1016/j.urolonc.2014.05.002
- [23] Chandrasekar T, Boorjian SA, Capitanio U, Gershman B, Mir MC, Kutikov A. Collaborative review: factors influencing treatment decisions for patients with a localized solid renal mass. Eur Urol. 2021;80(5):575–88. https://doi.org/10.1016/j.eururo.2021.01.021
- [24] Yap SA, Finelli A, Urbach DR, Tomlinson GA, Alibhai SM. Partial nephrectomy for the treatment of renal cell carcinoma (RCC) and the risk of end-stage renal disease (ESRD). BJU Int. 2015;115(6):897–906. https://doi.org/10.1111/bju.12883
- [25] Lane BR, Campbell SC, Demirjian S, Fergany AF. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189(5):1649–55. https://doi.org/10.1016/j.juro.2012.11.121
- [26] Wang S, Liu Z, Zhang D, Xiang F, Zheng W. The incidence and risk factors of chronic kidney disease after radical nephrectomy in patients with renal cell carcinoma. BMC Cancer. 2022;22(1):1138. https://doi.org/10.1186/s12885-022-10245-8
- [27] Wu J, Suk-Ouichai C, Dong W, Antonio EC, Derweesh IH, Lane BR, et al. Analysis of survival for patients with chronic kidney disease primarily related to renal cancer surgery. BJU Int. 2018;121(1):93–100. https://doi.org/10.1111/bju.13994
- [28] Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol. 2009;182(2):435–43; discussion 43–4. https://doi.org/10.1016/j.juro.2009.04.004
- [29] Bhindi B, Asante D, Branda ME, Hickson LJ, Mason RJ, Jeffery MM, et al. Survival outcomes for patients with surgically induced end-stage renal disease. Can Urol Assoc J. 2020;14(3):E65–73. https://doi.org/10.5489/cuaj.6010
- [30] Landberg A, Bruce D, Lindblad P, Ljungberg B, Lundstam S, Thorstenson A, et al. Validation of data quality in the National Swedish Kidney Cancer Register. Scand J Urol. 2021;55(2):142–8. https://doi.org/10.1080/21681805.2021.1885485