ORIGINAL RESEARCH ARTICLE
Evaluation of data quality in the Swedish National Penile Cancer Register
Åsa Warnolfa,b* , Dominik Glombikc*
, Dominik Glombikc* , Fredrik Sandind, Mats Lambee
, Fredrik Sandind, Mats Lambee , Gediminas Baseckasb, Axel Gerdtssona,b
, Gediminas Baseckasb, Axel Gerdtssona,b , Kimia Kohestanif,g*
, Kimia Kohestanif,g* and Peter Kirranderc*
and Peter Kirranderc*
aDepartment of Translational Medicine, Lund University, Malmö, Sweden; bDepartment of Urology, Skåne University Hospital, Malmö, Sweden; cDepartment of Urology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden; dRegional Cancer Centre Central-Sweden, Uppsala, Sweden; eDepartment of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; fDepartment of Urology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; gDepartment of Urology, Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden
ABSTRACT
Objective: The National Penile Cancer Register (NPECR) in Sweden was initiated in year 2000 and currently contains more than 3,900 men diagnosed with penile cancer. The aim of this study was to evaluate data quality in the NPECR in terms of completeness, timeliness, comparability, and validity.
Material and methods: Completeness was assessed by cross-linkage to the Swedish Cancer Register. Timeliness, defined as time from date of diagnosis to date of reporting in the NPECR, was calculated. Comparability was evaluated by reviewing and comparing coding routines in the NPECR with national and international guidelines. To assess validity, medical records of 375 men with a penile cancer diagnosis in the NPECR between 2017 and 2020 were reviewed and selected variables were re-abstracted and compared with previously registered data.
Results: Completeness was high (93%). Timeliness was in median 4.6 (Inter Quartile Range 2.6–8.8) months. Comparability was good with coding routines and the registration forms were in compliance with current guidelines. Overall, the validity was high. The majority of variables showed an exact agreement exceeding 90%.
Conclusion: Data quality in the Swedish NPECR is generally high with respect to completeness, timeliness, comparability, and validity. Hence, the NPECR represents a reliable data source for monitoring the quality of penile cancer care and research. Data quality can be further improved by revision of reporting forms and manuals, training of reporting staff, and by organizational adjustments.
KEYWORDS: Penile cancer; quality register; validation; Sweden
Citation: Scandinavian Journal of Urology 2024, VOL. 59, 162–168. https://doi.org/10.2340/sju.v59.42029.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 29 May 2024; Accepted: 13 September 2024; Published: 2 October 2024
CONTACT Åsa Warnolf asa.warnolf@med.lu.se Department of Urology, Skåne University Hospital, Department of Translational Medicine, Lund University, Jan Waldenströms gata 5, SE-205 02 Malmö, Sweden
**D.G. and Å.W. together with K.K. and P.K. contributed equally to this work.
Competing interests and funding: The authors declare that they have no conflict of interest.
Introduction
The Swedish Cancer Register (SCR), to which reporting is mandated by law [1], is used to monitor the incidence of cancer, but lacks information on treatment and follow-up. As a complement, Sweden has a long tradition of clinical quality registers that collect these clinical data. In 2023, there were more than 100 different clinical quality registers, including 30 national cancer quality registers [2]. Data collected in the National cancer quality registers are used for evaluation of health care quality, benchmarking, and register-based research. The National Penile Cancer Register (NPECR) was founded in year 2000 to monitor penile cancer care in Sweden. The NPECR is population-based and currently includes more than 3,900 men with penile cancer with information on diagnosis, surgical-, dermatological-, and oncological treatment, and follow-up data [1, 3].
The web-based platform INCA (Information Network for Cancer) is used for online reporting to the NPECR. At the time of launch of the NPECR, a combined form registering information on TNM classification and primary treatment was used. Over time, the forms have been modified, and now divided into registration, work-up and planned treatment, and surgical treatment forms, respectively. In 2015, new forms for oncological treatment with chemo- and radiotherapy and follow-up at 2 and 5 years were introduced. The Regional Cancer Center in Central Sweden provides national, technical and administrative support to the NPECR, while six regional penile cancer centers across Sweden are responsible for the completeness of the reported data.
Due to the rarity of penile cancer, the organization of care has undergone several changes during the last decade. A weekly national multidisciplinary team (MDT) conference was introduced in 2013 and in 2015 curative surgery was centralized to two national centers, Skåne University Hospital and Örebro University Hospital, to ensure an improved and equal provision of care for men with penile cancer. Clinical work-up and follow-up is mainly (but not exclusively) carried out at the six regional penile cancer centers, one in each Swedish health care region. The two national centers also constitute regional centers for their respective health care regions.
The aim of this study was to evaluate data quality in the NPECR in terms of completeness, timeliness, comparability, and validity.
Material and methods
The quality of data collected in the NPECR was evaluated according to the validation strategy originally described by Parkin and Bray [4], which includes four dimensions of register data quality: completeness, timeliness, comparability, and validity. A validation manual for cancer quality registers on the INCA platform was used as a guiding document [5]. The study period was set to 2017–2020, to focus on the current centralized organization of penile cancer care in Sweden and with the present TNM classification and forms for registration to the NPECR. Since 2017, the 8th edition of the TNM Classification of Malignant Tumors has been used [6].
Completeness
Completeness was calculated as the proportion of cases registered in the NPECR compared to all penile cancer cases registered in the SCR [4] during the period under study.
Timeliness
Timeliness was defined as the time between the date of penile cancer diagnosis and the actual date of registration according to the NPECR [4].
Comparability
Comparability was assessed by evaluating if the registration and coding routines are clear, nationally uniform, and whether they follow national [7] and international guidelines [8], hence enabling national and international comparisons [4]. Comparability was evaluated based on the registration and coding routines applied during the review period.
Validity
Validity was defined as the proportion of cases in the data set with a specific characteristic that truly had the characteristic in question [4]. Re-abstraction of data from medical records was used as validation method and included independent review of information from original patient records and subsequent comparisons with data registered in the NPECR. To ensure nationwide representation, patients from all six regional penile cancer centers were included in the validation process. The final data set comprised 375 men with penile cancer after exclusion of 13 men due to previous incorrect registration or opt-out of the NPECR (Figure 1). Based on assessment of importance and clinical relevance, 31 (out of 289) specific variables were selected from the forms for registration, work-up and planned treatment, surgical treatment, and follow-up form, respectively.

Figure 1. Regional penile cancer centers with number of cases validated during the study period 2017–2020. Skåne University Hospital and Örebro University Hospital constitute both regional and national centers for penile cancer in Sweden.
The re-abstracted data were registered on the INCA platform by use of a form specifically designed for the validation. Retrieval of medical records and data re-abstraction was performed by a nurse with experience of Swedish health care and administration of cancer quality registers, including the NPECR specifically. At the time of the validation process, the validator was not affiliated with any of the reporting units.
Validity was analyzed by calculating the exact agreement between the data recorded in the NPECR and the data re-abstracted from medical records. Strength of agreement was measured by Cohen’s Kappa for categorical variables and Pearson correlation coefficient for numerical variables.
The study was approved by the Regional Ethics Committee in Uppsala (2023-05066-02).
Results
Completeness
The mean national level of completeness during the study period was 93% with a range of 80% – 99% between the six health care regions.
Timeliness
The median time between date of diagnosis and registration in the NPECR was 4.6 months (Inter Quartile Range (IQR) 2.6–8.8). The cumulative proportion of individuals reported to the NPECR was 32% within 3 months, 62% within 6 months, and 83% within 12 months (Figure 2).
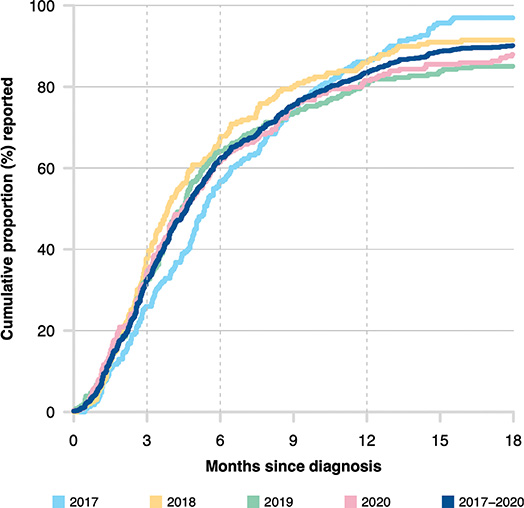
Figure 2. Timeliness for registration in the National Penile Cancer Register (NPECR) between 2017 and 2020.
Comparability
The coding routines and the forms for registration were reviewed and are consistent with both national and European guidelines for penile cancer [7, 8]. The NPECR is updated regularly, including complying with the updates in TNM classification [6]. Updated versions of the reporting forms are available online, although reporting is mainly performed on the web-based INCA platform [9].
Validity
The results of the validation of the 31 selected variables from the NPECR are summarized in Table 1. In general, the proportions of exact agreement between the NPECR and re-abstraction data set were high for all 31 variables, ranging from 66% to 100%; however, larger variations were seen in the correlation coefficients (Table 1).
| Variable | Number (%) of complete records in register data | Number (%) of complete records in validation data | Number (%) of complete records in both | Number (%) of exact agreement* | Correlation** | |||||
| Registration form | ||||||||||
| Date of diagnosis | 375 | (100) | 374 | (100) | 374 | (100) | 245 | (66) | 0.98 | (P) |
| cT stage | 373 | (99) | 375 | (100) | 373 | (99) | 261 | (70) | 0.60 | (C) |
| cN stage | 371 | (99) | 372 | (99) | 368 | (98) | 287 | (78) | 0.34 | (C) |
| Has the patient been assigned a navigator nurse at the diagnostic clinic? | 358 | (95) | 288 | (77) | 279 | (74) | 215 | (77) | 0.49 | (C) |
| Workup and planned treatment form | ||||||||||
| Has the patient been discussed at a national multidisciplinary conference? | 369 | (98) | 367 | (98) | 361 | (96) | 337 | (93) | 0.55 | (C) |
| Has the patient been treated at one of the national centers? | 367 | (98) | 369 | (98) | 361 | (96) | 343 | (95) | 0.87 | (C) |
| Has the patient been assigned a navigator nurse at the treating clinic? | 348 | (93) | 369 | (98) | 344 | (92) | 293 | (85) | 0.48 | (C) |
| Radiological primary investigation | 371 | (99) | 369 | (98) | 365 | (97) | 344 | (94) | 0.88 | (C) |
| Radiotherapy | 357 | (95) | 369 | (98) | 353 | (94) | 342 | (97) | 0.46 | (C) |
| Chemotherapy | 357 | (95) | 369 | (98) | 353 | (94) | 345 | (98) | 0.72 | (C) |
| Surgical treament form | ||||||||||
| Local excision | 352 | (94) | 342 | (91) | 329 | (88) | 292 | (89) | 0.75 | (C) |
| Laser surgery | 352 | (94) | 342 | (91) | 329 | (88) | 326 | (99) | 0.72 | (C) |
| Glansectomy | 352 | (94) | 342 | (91) | 329 | (88) | 317 | (96) | 0.81 | (C) |
| Glansectomy+neo-glans | 352 | (94) | 342 | (91) | 329 | (88) | 328 | (100) | 0.95 | (C) |
| Circumcision | 352 | (94) | 341 | (91) | 328 | (87) | 296 | (90) | 0.77 | (C) |
| Urethrectomy | 352 | (94) | 341 | (91) | 328 | (87) | 324 | (99) | 0.83 | (C) |
| Glans resurfacing | 352 | (94) | 342 | (91) | 329 | (88) | 326 | (99) | 0.85 | (C) |
| Partial amputation | 352 | (94) | 343 | (91) | 330 | (88) | 321 | (97) | 0.92 | (C) |
| Total amputation | 352 | (94) | 342 | (91) | 329 | (88) | 327 | (99) | 0.97 | (C) |
| pT stage | 352 | (94) | 343 | (91) | 330 | (88) | 294 | (89) | 0.86 | (C) |
| Grade | 352 | (94) | 318 | (85) | 307 | (82) | 264 | (86) | 0.81 | (C) |
| Tumor-free resection margins | 347 | (93) | 336 | (90) | 319 | (85) | 270 | (85) | 0.37 | (C) |
| Lymph node examination | 352 | (94) | 341 | (91) | 328 | (87) | 313 | (95) | 0.90 | (C) |
| pN stage | 352 | (94) | 341 | (91) | 328 | (87) | 301 | (92) | 0.87 | (C) |
| Sentinel node | 352 | (94) | 343 | (91) | 330 | (88) | 315 | (95) | 0.91 | (C) |
| Lymph node dissection: Inguinal | 352 | (94) | 342 | (91) | 329 | (88) | 318 | (97) | 0.87 | (C) |
| Lymph node dissection: Pelvic | 352 | (94) | 342 | (91) | 329 | (88) | 327 | (99) | 0.93 | (C) |
| Lymph node dissection: Other | 352 | (94) | 342 | (91) | 329 | (88) | 324 | (98) | 0.54 | (C) |
| Follow-up form | ||||||||||
| Local recurrence after primary treatment | 77 | (48) | 162 | (100) | 77 | (48) | 72 | (94) | 0.72 | (C) |
| Regional metastases after primary treatment | 77 | (48) | 161 | (99) | 76 | (47) | 73 | (96) | 0.78 | (C) |
| Distans metastases after primary treatment | 77 | (48) | 161 | (99) | 76 | (47) | 74 | (97) | 0.79 | (C) |
| *Among those with complete records in both register and validation data. | ||||||||||
| **Pearson correlation coefficient (P) for numeric variables, Cohen’s kappa (C) for ordinal variables. | ||||||||||
Seven high impact variables with representation from different registration forms were selected for detailed review. The results are presented next with additional information available in Figures 2 and 3.

Figure 3. (a–f) Agreement between data registered in the National Penile Cancer Register (NPECR) and data re-abstracted from medical records (selected variables).
Date of diagnosis
The variable ‘Date of diagnosis’ was 100% complete in both the NPECR and the re-abstracted data set. The exact agreement was 66% while the correlation was 0.98 (Table 1). The discrepancy reflects that even a 1-day difference was considered discrepant, yet the correlation remains high (Table 1).
cT-stage and pT-stage
The variable clinical tumor stage, ‘cT-stage’, was complete in 99% and 100% in the NPECR and the re-abstracted data set, respectively. The exact agreement was 70% and the correlation 0.6. The pathological tumor stage, ‘pT-stage’, was complete in 94% and 91%, respectively. The exact agreement was 89% with a correlation coefficient of 0.86 (Figure 3a and b).
Tumor-free resection margins
The variable ‘Tumor-free resection margins’ was 93% complete in the NPECR and 90% in the re-abstracted data set. The exact agreement regarding ‘Tumor-free resection margins’ was 85% while the correlation was 0.37. Out of 271 men registered in the NPECR with negative resection margins, 252 (93%) were classified accordingly in the re-abstracted data. In the NPECR, resection margins were registered as negative for eight individuals, but as positive during the re-abstraction. The patient records of 11 individuals were missing. Conversely, 27 of 45 men (60%) with a record of positive resection margins in the NPECR had negative resection margins according to the re-abstracted data (Figure 3c).
Lymph node evaluation and pN-stage
The variable ‘Evaluation of lymph nodes’ in the NPECR includes undergoing any or a combination of dynamic sentinel node biopsy, modified and radical inguinal lymph node dissection. The exact agreement regarding this variable between the NPECR and the re-abstracted data set was 95% with a Cohen’s kappa of 0.9. The findings for the variable ‘pN-stage’ were similar, with an exact agreement of 92% and a correlation coefficient of 0.87 for men with a pN-stage available in both the NPECR and the re-abstracted data set (Figure 3d and e).
Local recurrence
The variable ‘Local recurrence’ had a low proportion of complete records in the NPECR (48%) at 2-year follow-up. The correlation between the NPECR data and re-abstracted data was 0.72 (Figure 3f).
Discussion
The NPECR is a unique database used both for monitoring the quality of penile cancer care and research. To the best of our knowledge, it represents the largest population-based penile cancer register in the world. The aim of this study was to evaluate the data quality in the NPECR in terms of completeness, timeliness, comparability, and validity. All registration forms were reviewed and variables with the highest clinical relevance were selected for detailed assessment. Our study shows that the data quality in the NPECR generally is high with respect to all these four dimensions of register data quality. Moreover, difficult-to-interpret clinical variables and those with a low degree of completeness were identified during validation process.
By cross-linking data in the NPECR (for the years 2000–2012) with information in several other Swedish nationwide registers, a research database Penile Cancer Database Sweden (PenCBaSe) has been generated. The PenCBaSe has resulted in several publications that have improved the understanding of penile cancer and potentially also care and treatment [10–14]. The PenCBaSe is currently being updated to include all men registered in the NPECR until 2023.
Internationally, prospective population-based quality registers on penile cancer are rare. In Denmark, the Danish National Penile Cancer Quality database was launched in 2011 [15]. A recent publication by Vreeburg et al. described penile cancer care based on information in the Netherlands Cancer Registry (NCR) including all men diagnosed with penile cancer since 1990 [16]. In other countries, information on men with penile cancer has been collected retrospectively in hospitals or from national registers with limited or no follow-up data available [17, 18].
The main strength of the NPECR is its high completeness, continuously increasing as delayed registrations are being added over time. During the period under study, 2017–2020, the completeness was 93% compared to 98% when assessed for calendar years 2006–2020 [3].
The proportion of men reported to the NPECR within 12 months of diagnosis (83% for 2017–2020) was lower compared to other Swedish cancer quality registers. The corresponding estimate was 96% in the National Kidney Cancer Register, 98% in the National Breast Cancer Register, and 98% in the National Prostate Cancer Register [19]. Thus, additional measures to improve the timeliness of reporting to the NPECR are needed. This could include direct reporting in conjunction with the national weekly MDT conference.
Regarding comparability, we conclude that the coding routines and registration forms were consistent with current guidelines, ensuring that the data can be used for national and international comparisons.
The large geographical distance between reporting clinics, some of which only report a few cases over a period of several years, prevented inclusion of all newly diagnosed penile cancer cases during the period chosen for the validation process. However, to guarantee a nationwide representation, men with penile cancer reported to the NPECR by all six regional penile cancer centers were included. To ensure a uniform validation process of high quality, the work was performed on-site at each regional center by an experienced nurse validator. Considering the latest changes of the penile cancer care in Sweden, the study period from 2017 to 2020 was chosen to reflect its current organization with the latest TNM classification and registration forms.
The exact agreement of cT-stage reached only 70%. One possible explanation is that the registration and assessment of cT-stage is often made by local urologists with limited experience of penile cancer. In general, the first assessment is heavily influenced by biopsy results that may not be representative of the whole tumor. However, at re-abstraction additional clinical information may have become available. There are ongoing discussions, whether cT-stage should be updated and registered, in the NPECR, in conjunction with the national weekly MDT conference, when photography, status description, and pathology report of biopsies are reviewed by experienced and dedicated urologists, dermatologists, and pathologists. As a direct consequence of the validation process, cT-stage reassessment is now a mandatory part of the national weekly MDT conference where it is explicitly expressed and registered in the MDT report.
The kappa score regarding regarding tumor-free resection margins was low, as 27 out of 45 men who were considered having positive margins in the register had negative margins according to the re-abstraction. Consequently, an update of the manual of the NPECR is currently discussed, more specifically, where PeIN at the resection margin should not be considered to constitute a positive margin when the invasive cancer is radically removed.
The most important predictor of penile cancer survival is lymph node involvement [20]. The variables on pN-stage and lymph node evaluation both showed high correlation and high exact agreement, 92% and 95%, respectively. The somewhat lower agreement regarding pN-stage found in our study might be due to changes in pN stage definitions between the 7th and the 8th edition of the TNM classification, a change that was not completely implemented by all reporting units at the beginning of the study period.
Registration of recurrences may be hampered by the current organization of Swedish penile cancer care with surgery being performed at one of the two national centers, while follow-up is carried out mostly at regional centers or local hospitals with limited resources.
Conclusion
Data quality in the Swedish NPECR is generally high with respect to completeness, timeliness, comparability, and validity. The results of this study show that the NPECR is a reliable data source for the monitoring of penile cancer care and research. Data quality can be further improved by revision of reporting forms and manuals, training of reporting staff, and by implementing direct reporting in conjunction with the national weekly MDT conference.
Acknowledgments
The study was made possible by the continuous work of the NPECR steering committee. This work was supported by Gösta Jönsson Research Foundation, the Foundation of Urological Research, and Hillevi Fries Research Foundation. The funding sources had no role in the study design, data analyses, interpretation of the results, or writing of the manuscript.
Our special thanks go to Gun-Britt Adamsson, the validator, for her contribution and making this validation possible.
Data availability statement
The findings of this study are mainly based on data in the NPECR. Legal restrictions prohibit public data sharing of the data set because it includes information provided by third parties. Because of the large number of variables, the data set is not considered to be fully anonymized. According to Swedish law, the following restrictions therefore apply: we are not allowed to share data on individual study subjects with other researchers, nor to upload such data on an open server. However, on demand, access to the research data set can be provided on a remote server where analyses can be performed and aggregated data in Figures and Tables can be exported, but no data on individual participants can be downloaded. Researchers can apply for access by contacting the steering group of the NPECR and the data access committee at the Regional Cancer Center Central-Sweden by e-mail to: datauttag-rcc@rccmellan.se. After approval, a study file will be uploaded to a remote access server. Users will be charged for software licenses, administration, and data management.
ORCID
Åsa Warnolf  https://orcid.org/0009-0003-1282-8024
https://orcid.org/0009-0003-1282-8024
Dominik Glombik  https://orcid.org/0009-0007-9517-4773
https://orcid.org/0009-0007-9517-4773
Mats Lambe  https://orcid.org/0000-0002-4624-3767
https://orcid.org/0000-0002-4624-3767
Axel Gerdtsson  https://orcid.org/0000-0003-4033-5078
https://orcid.org/0000-0003-4033-5078
Kimia Kohestani  https://orcid.org/0000-0003-0244-5508
https://orcid.org/0000-0003-0244-5508
Peter Kirrander  https://orcid.org/0000-0002-4738-9223
https://orcid.org/0000-0002-4738-9223
References
- [1] Nationell_kvalitetsregistergrupp_peniscancer. Peniscancer: Nationell kvalitetsrapport för 2022 [Internet]. 2023 [cited 07.10.2023]. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/peniscancer/kvalitetsregister/20231003_npecr_nationell_rapport_2022.pdf
- [2] Sveriges_kommuner_och_regioner. National quality registries in Sweden [Internet]. 2023 [cited 27.09.2023]. Available from: https://skr.se/en/kvalitetsregister/omnationellakvalitetsregister.52218.html
- [3] Nationella_peniscancerregistret_(NPECR). Interaktiv onlinerapport peniscancer [Internet]. [cited 27.09.2023]. Available from: https://statistik.incanet.se/peniscancer/
- [4] Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer. 2009;45(5):747–755. https://doi.org/10.1016/j.ejca.2008.11.032
- [5] Information_från_arbetsgruppen_för_kvalitetsregister_och_INCA. Validering av kvalitetsregister på INCA: Version 3.0 [Internet]. Available from: https://cancercentrum.se/globalassets/vara-uppdrag/kunskapsstyrning/kvalitetsregister/validering/manual-for-validering-av-kvalitetsregister-inom-cancer.pdf
- [6] Sanchez DF, Fernandez-Nestosa MJ, Cañete-Portillo S, et al. Evolving insights into penile cancer pathology and the eighth edition of the AJCC TNM staging system. Urol Oncol. 2022;40(6):215–222. https://doi.org/10.1016/j.urolonc.2020.09.010
- [7] Regionala_cancercentrum_i_samverkan. Nationellt vårdprogram Peniscancer [Internet]. 2023 [cited 27.09.2023]. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/peniscancer/vardprogram/
- [8] Brouwer OR, Albersen M, Parnham A, et al. European Association of Urology-American Society of Clinical Oncology collaborative guideline on penile cancer: 2023 update. Eur Urol. 2023;83(6):548–560. https://doi.org/10.1016/j.eururo.2023.02.027
- [9] Nationell_kvalitetsregistergrupp_peniscancer. Nationellt_kvalitetsregister_för_peniscancer [Internet]. 2024 [cited 08.04.2024]. Available from: https://cancercentrum.se/samverkan/cancerdiagnoser/penis/kvalitetsregister/dokument/
- [10] Torbrand C, Wigertz A, Drevin L, et al. Socioeconomic factors and penile cancer risk and mortality; a population-based study. BJU Int. 2017;119(2):254–260. https://doi.org/10.1111/bju.13534
- [11] Kristiansen S, Svensson Å, Drevin L, et al. Risk factors for penile intraepithelial neoplasia: a population-based register study in Sweden, 2000–2012. Acta Derm Venereol. 2019;99(3):315–320. https://doi.org/10.2340/00015555-3083
- [12] Kristiansen S, Bjartling C, Svensson A, et al. Penile intraepithelial neoplasia, penile cancer precursors and human papillomavirus prevalence in symptomatic preputium: a cross-sectional study of 351 circumcised men in Sweden. BJU Int. 2021;127(4):428–434. https://doi.org/10.1111/bju.15221
- [13] Glombik D, Oxelbark Å, Sundqvist P, et al. Risk of second HPV-associated cancers in men with penile cancer. Acta Oncol. 2021;60(5):667–671. https://doi.org/10.1080/0284186X.2021.1885056
- [14] Glombik D, Davidsson S, Sandin F, et al. Penile cancer: long-term infectious and thromboembolic complications following lymph node dissection – a population-based study (Sweden). Acta Oncol. 2023;62(5):458–464. https://doi.org/10.1080/0284186X.2023.2206524
- [15] Jakobsen JK, Ozturk B, Sogaard M. The Danish National Penile Cancer Quality database. Clin Epidemiol. 2016;8:589–594. https://doi.org/10.2147/CLEP.S99513
- [16] Vreeburg MTA, de Vries HM, van der Noort V, et al. Penile cancer care in the Netherlands: increased incidence, centralisation, and improved survival. BJU Int. 2024;133(5):596–603. https://doi.org/10.1111/bju.16306
- [17] Borque-Fernando Á, Gaya JM, Esteban-Escaño LM, et al. Epidemiology, diagnosis and management of penile cancer: results from the Spanish National Registry of Penile Cancer. Cancers (Basel). 2023;15(3):616. https://doi.org/10.3390/cancers15030616
- [18] Deng X, Liu Y, Zhan X, et al. Trends in incidence, mortality, and survival of penile cancer in the United States: A population-based study. Front Oncol. 2022;12:891623. https://doi.org/10.3389/fonc.2022.891623
- [19] Regionala_cancercentrum_i_samverkan. Nationella kvalitetsregister cancer [Internet]. Available from: https://cancercentrum.se/samverkan/vara-uppdrag/kunskapsstyrning/kvalitetsregister/
- [20] Sachdeva A, McGuinness L, Zapala L, et al. Management of lymph node-positive penile cancer: a systematic review. Eur Urol. 2024;85(3):257–273. https://doi.org/10.1016/j.eururo.2023.04.018 [cited 08.04.2024]