ORIGINAL RESEARCH ARTICLE
Initial management and survival of patients with primary metastatic bladder cancer before the immunotherapy era: a population-based study from Norway
Christina Tanem Møllera,b , Gunnar Tafjordc
, Gunnar Tafjordc , Augun Blindheimd,e
, Augun Blindheimd,e , Viktor Bergeb,f
, Viktor Bergeb,f , Sophie D Fossåb,g,+
, Sophie D Fossåb,g,+ and Bettina Kulle Andreassena,+
and Bettina Kulle Andreassena,+
aDepartment of Research, Cancer Registry of Norway, Oslo, Norway; bFaculty of Medicine, University of Oslo, Oslo, Norway; cDepartment of Oncology, Oslo University Hospital, Oslo, Norway; dDepartment of Clinical and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway; eDepartment of Surgery, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; fDepartment of Urology, Oslo University Hospital, Oslo, Norway; gNational Advisory Unit on Late Effects after Cancer Treatment, Oslo University Hospital, Oslo, Norway
ABSTRACT
Before immunotherapy became part of the management of metastatic bladder cancer (mBC), systemic anti-cancer treatment comprised primarily of platinum-based chemotherapy. The objective of this study was to describe the characteristics, the initial management, overall survival (OS) and hospitalisations of patients with mBC before 2018 when immunotherapy for mBC was introduced in Norway.
Material and methods: It is a nationwide population-based study of primary mBC patients (diagnosed 2008-16). Descriptive statistics were applied and stratified for four initial management options (≤150 days after BC diagnosis): chemotherapy, major local treatment (cystectomy/pelvic radiotherapy), multimodal treatment (chemotherapy and local) and no anti-cancer treatment beyond transurethral resection of bladder tumour (untreated). Group differences were evaluated by Chi-square and Kruskal–Wallis test; OS was estimated with Kaplan–Meier.
Results: Of the 305 patients included, 76 (25%) patients had chemotherapy, 46 (15%) patients had major local treatment, 21 (7%) patients had multimodal treatment and 162 (53%) patients were untreated. Median OS ranged from 2.3 months (untreated) to 9.8 months (chemotherapy). Patients who received treatment had a higher rate of hospitalisation, with a median stay of three to four times that of untreated patients.
Conclusion: Before immunotherapy, more than 50% of patients with primary mBC did not receive any initial anti-cancer therapy and had a poor survival. Patients treated with chemotherapy had inferior median OS compared to those treated with comparable systemic strategies in contemporary trials. Our results provide a basis for future research on treatment and survival after the introduction of immunotherapy for mBC, aiming to improve the care and outcome of patients with mBC.
KEYWORDS: Survival; metastatic bladder cancer; preimmunotherapy; population based; chemotherapy
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 101–108. https://doi.org/10.2340/sju.v58.5923.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 13 December 2022; Accepted: 8 September 2023; Published: 10 November 2023
*CONTACT Christina Tanem Møller Christina Tanem.Moller@kreftregisteret.no Department of Research, Cancer Registry of Norway, Pb 5313 Majorstuen, 0304 Oslo, Norway
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v58.5923
+Shared last authorship.
Competing interests and funding: The authors report no conflicts of interest.
This work was supported by the Dam Foundation (https://dam.no) under grant number 2019/FO249584 and Radiumhospitalets Legater (https://radiumlegat.no).
Introduction
In metastatic bladder cancer (mBC) management, platinum-based combination chemotherapy is the guideline-recommended first-line treatment [1, 2]. This recommendation has remained unchanged since pivotal trials were published more than 20 years ago [3–5]. Approximately 50% of patients are ineligible for cisplatin due to impaired renal function, heart failure or poor performance status [6, 7], though carboplatin can sometimes be offered as an alternative [8]. Recently, novel agents were approved for use in the management of mBC. In 2017/2018, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved three immune check-point inhibitors (ICIs) [9–11], and an additional ICI and a novel antibody drug conjugate were approved in 2021 [12–14]. Current European guidelines recommend maintenance ICI after first-line platinum-based chemotherapy in patients with stable disease, ICIs as standard second-line therapy and an antibody drug conjugate as third-line therapy [1, 2].
In Norway, 1,659 patients were diagnosed with bladder cancer (BC) in 2021, of which approximately 5% of patients presented with metastases [15]. Norwegian guidelines for the treatment of mBC are in line with European guidelines for treatment [16, 17], and eligible patients are treated with first-line platinum-based chemotherapy. ICIs as standard second-line treatment were approved for use in Norway in 2018. However, antibody drug conjugates are not yet approved.
Real-world studies describing the pre-immunotherapy management and outcomes of patients with mBC are needed as references for upcoming studies of novel agents in routine clinical practice. To our knowledge, there are no Norwegian studies describing the initial management, survival and healthcare use of patients with mBC. Moreover, according to previously published population-based studies, a large proportion of patients (60% – 65%) are left untreated by chemotherapy [18–20]. Characteristics and survival of these patients have not been well described in the literature.
Thus, in this Norwegian population-based study, we aimed to describe the characteristics of patients with primary mBC and their initial management, overall survival (OS) and the burden of hospitalisations from the date of diagnosis until end of follow-up.
Material and methods
Data sources
The Cancer Registry of Norway (CRN) is a national cancer registry established in 1951. Notification of all new cancer cases to the CRN is compulsory by law [15], and the data are considered near-complete, accurate and internationally comparable [21]. Information about age, sex, health region, date of BC diagnosis, morphology and metastases is available. Clinical tumour category (T-category) was not available. Metastases (regional and non-regional lymph nodes and pelvic and non-pelvic visceral metastases) are registered as present at the time of a first-time cancer diagnosis if discovered within the diagnostic period, defined by the CRN as ≤150 days since the first histological verification of cancer. In addition, the CRN contains information about surgery (histology reports), radiotherapy (RT) and causes of death with corresponding dates.
The CRN data are regularly validated by the data in the Norwegian Patient Registry (NPR). The NPR is a mandatory population-based registry, which covers all public specialist health-care services in Norway [22, 23]. Information from the NPR was linked to data from CRN by the personal identification number assigned to all new-borns and residents in Norway since 1960. Data on individual administrative, demographic and coded medical information (diagnoses, surgical and medical procedures, and chemotherapy) from all patients’ contacts with public hospitals from 2008 and onward are registered in the NPR.
Study population
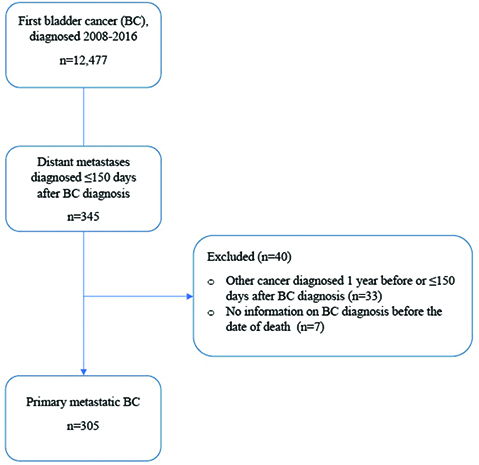
From the CRN, we selected all patients diagnosed between 2008 and 2016 with primary mBC (International Classification of Diseases [ICD]-10 C67). Primary mBC was defined as histologically verified BC (urothelial carcinoma [UC] and non-UC histology) registered with distant metastases during the diagnostic period as defined by the CRN. We considered non-regional lymph node metastases and metastases localised outside the true pelvis as distant metastases. Patients with regional lymph node metastases only were excluded. Patients with metastases diagnosed after the diagnostic period were not included due to incomplete registration in the CRN. Patients were excluded if another malignancy was diagnosed within 1 year prior to the mBC diagnosis or within the diagnostic period (≤150 days). We excluded patients with no information on BC diagnosis before the date of death.
Measures
From the CRN, the date of BC diagnosis was defined as the date of the first histologically verified diagnosis registered at the CRN. Year of BC diagnosis was categorised in three periods (2008–2010, 2011–2013 and 2014–2016). Place of residence was categorised according to the four official health regions in Norway (Southeast, West, Central and North). Surgical procedures (transurethral resection of bladder tumour [TURBT] and cystectomy) and the application of RT registered with the ICD-10 code C67 were identified. Pelvic radiotherapy (PRT) was defined as RT of pelvic soft tissue tumour manifestations. We defined the underlying cause of death to be BC if registered with ICD-10 code C67, C68 (unspecified urinary tract) or C80 (unspecified location of malignant tumour).
From the NPR, information on TURBTs and cystectomy from the CRN was cross-checked to identify and include unreported procedures. Chemotherapy was identified by procedure codes for intravenous administration with or without specified drug codes for platinum-based chemotherapy for BC (cisplatin or carboplatin). After the BC diagnosis, we considered all chemotherapy provided to patients with ICD-10 codes C65-C68 (urinary tract cancer), C80 and C77-C79 (metastases) as chemotherapy treatments for BC. In the NPR, the patients are categorised according to the type of hospital contact: day-patient, outpatient or inpatient, with corresponding dates for admission and discharge. Our term ‘hospitalisation’ considers only inpatient contacts of any cause after BC diagnosis. For each individual hospitalisation, we calculated the interval number of days from hospital admittance to discharge (days of hospitalisation). We then summarised the days of hospitalisation for each patient within the follow-up time (total days of hospitalisation per patient).
The initial management was defined as whether patients received any anti-cancer treatment (yes or no) and also the type of treatment that patients received (systemic and/or local). Initial management was commenced within the diagnostic period (≤150 days) after TURBT confirming BC diagnosis. Patients were allocated into four categories as described in Table 1. Patients had to receive at least one chemotherapy administration to be considered as recipients of chemotherapy. Both local therapies after initial management and subsequent lines of systemic anti-cancer therapy after the diagnostic period were not included in our analysis.
| Group | Initial management |
| 1 | Chemotherapy, without any local treatment (cystectomy, pelvic radiotherapy, TURBT*) (‘chemo’) |
| 2 | Major local treatment without use of chemotherapy: cystectomy or pelvic radiotherapy (‘local’) |
| 3 | Chemotherapy in combination with any local treatment** or combination of local treatments (‘multimodal’) |
| 4 | No chemotherapy and no local treatments except TURBT only (‘untreated’) |
| *TURBT: transurethral resection of bladder tumour; **Includes peri-operative chemotherapy. | |
Statistical methods
Patient and treatment characteristics are presented applying descriptive statistics (median, interquartile range [IQR] and proportions). Distributions of variables between treatment groups were compared with Chi-square test for categorical variables and Kruskal–Wallis equality of populations rank test for continuous variables. The statistical significance level was set to ≤0.05.
Patients were followed from the date of BC diagnosis until the date of death, migration or the end of follow-up (December 31, 2019) whichever came first. Time in years from date of BC diagnosis was used as timescale in all analyses. Unadjusted survival curves (Kaplan Meier) display OS from the diagnosis to the end of follow-up.
Statistical analyses were performed using Stata 17 (StataCorp, College Station, TX).
Results
Patient characteristics
Out of the 12,477 patients with a BC diagnosis in 2008–2016, 345 (2.7%) patients were diagnosed with primary mBC, resulting in 305 evaluable patients (Figure 1). The median follow-up time was 154 days.
Median age at diagnosis was 73 years (Table 2), and most patients were male (69%). Women were older than men (median 76 vs. 72 years). Two patients were diagnosed with a second cancer (ICD-10 C65 and C34) after the diagnostic period (>150 days), and 48 (16%) patients had a history of previous cancer. The predominant histology was UC (70%). Distant metastases located exclusively in non-regional lymph node metastases were present in 38 (12%) patients. At the end of follow-up, 11 (4%) patients were still alive with BC being the cause of death in 255 (87%) out of 294 deaths. The characteristics of patients still alive at the end of follow-up are listed in Table S1.
| All | Chemo1 | Local2 | Multimodal3 | Untreated4 | Unadjusted P-value | |
| Patients, n (%) | 305 | 76 (25) | 46 (15) | 21 (7) | 162 (53) | |
| Days to primary treatment, median | 39 | 40 | 48 | 30 | N/A | P = 0.0096 |
| (IQR) | (29–70) | (28–71) | (33–75) | (24–41) | ||
| Age in years, median | 73 | 63 | 75 | 61 | 79 | P = 0.0001 |
| (IQR) | (65–82) | (57–69) | (67–82) | (50–69) | (73–86) | |
| Female (%) | 94 (31) | 22 (29) | 11 (24) | 6 (29) | 55 (34) | P = 0.583 |
| Health region (%) | P = 0.310 | |||||
| Southeast | 183 (100) | 42 (23) | 26 (14) | 13 (7) | 102 (56) | |
| West | 49 (100) | 10 (20) | 7 (14) | 2 (4) | 30 (61) | |
| Central | 39 (100) | 12 (31) | 5 (13) | 3 (8) | 19 (48) | |
| North | 31 (100) | 11 (35) | 8 (26) | 3 (10) | 9 (29) | |
| Missing | 3 (100) | 1 (33) | 0 | 0 | 2 (67) | |
| Previous non-BC cancer | 48 (16) | 13 (17) | 8 (18) | 1 (5) | 26 (16) | P = 0.548 |
| Year of BC diagnosis | P = 0.422 | |||||
| 2008–2010 | 112 (100) | 24 (21) | 13 (12) | 9 (8) | 66 (59) | |
| 2011–2013 | 101 (100) | 26 (26) | 21 (21) | 6 (6) | 48 (48) | |
| 2014–2016 | 92 (100) | 26 (28) | 12 (13) | 6 (7) | 48 (52) | |
| Histology | ||||||
| Urothelial carcinoma | 214 (70) | 54 (71) | 33 (72) | 17 (81) | 110 (68) | P = 0.649 |
| Metastases (exclusively) | P = 0.145 | |||||
| Lymph nodes, non-regional | 38 (12) | 15 (20) | 6 (13) | 2 (10) | 15 (9) | |
| Visceral | 267 (88) | 61 (80) | 40 (87) | 19 (90) | 147 (91) | |
| Number of deaths | 294 (96) | 71 (93) | 44 (96) | 20 (95) | 159 (98) | |
| Causes of death | ||||||
| Bladder cancer | 255 (87) | 69 (97) | 37 (84) | 19 (95) | 130 (82) | |
| Other cancer | 16 (5) | 1 (1) | 5 (11) | 1 (5) | 9 (6) | |
| Non-cancer cause | 23 (8) | 1 (1) | 2 (5) | 0 | 20 (12) | |
| 1Chemotherapy only. | ||||||
| 2Major local treatment (cystectomy or pelvic radiotherapy) only. | ||||||
| 3Chemotherapy in combination with any local treatment (cystectomy, pelvic radiotherapy, transurethral resection of bladder tumour (TURBT)) or a combination of local treatments. | ||||||
| 4No chemotherapy and no local treatments except TURBT only. | ||||||
Of the 305 patients included in the analysis, 76 (25%) received chemotherapy (chemo), 46 (15%) received major local therapy (local) with cystectomy (21 patients) or PRT (25 patients), 21 (7%) received multimodal treatment (multimodal) and 162 (53%) received no anti-cancer treatment (untreated) (Table 2). For the multimodal group, the treatment sequences by initial local or systemic treatment are shown in Table S2.
Time between BC diagnosis and start of initial treatment was shorter (median 1 month) for the multimodal group compared to the chemo and local groups where more than half of the treatments were initiated within 1.5 months after BC diagnosis (Table 2). In the untreated group, 16 patients had a second TURBT within the diagnostic period.
Univariable analyses showed that patients treated in the chemo and multimodal groups were younger than patients in the local and untreated groups (Table 2). Patients in the untreated group were more often women, residents of the Western health region and died more often of a non-cancer-related cause, compared to patients treated with local, multimodal or chemotherapy treatment. Lymph node metastases were more frequent in the chemo group compared to the other three groups.
Survival
Median OS for all patients with primary mBC was 5.1 months, and the 1, 3 and 5-year survival proportions were 23%, 10% and 8% (Figure 2a).
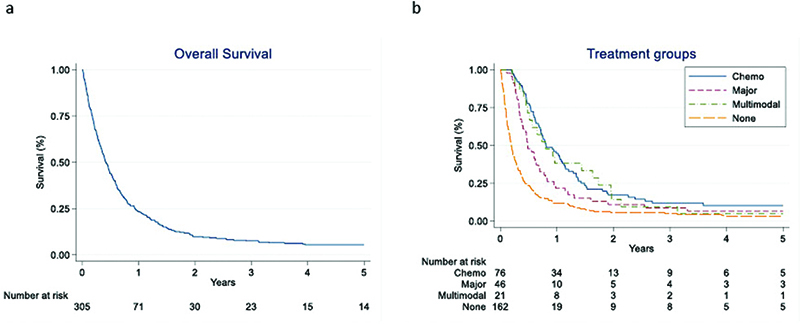
Figure 2. Patients with primary metastatic bladder cancer diagnosed between 2008 and 2016 in Norway: (a) Overall survival, (b) Overall survival stratified for treatment (Chemo: chemotherapy only; Local: Major local treatment [cystectomy or pelvic radiotherapy] only; Multimodal: Chemotherapy in combination with any local treatment (cystectomy, pelvic radiotherapy, transurethral resection of bladder tumour [TURBT]) or a combination of local treatments; Untreated: No chemotherapy and no local treatments except TURBT only).
Median OS was 9.8 months for patients in the chemo group, 5.9 months for patients in the local group, 9.7 months for patients in the multimodal group and 2.3 months for the patients in the untreated group (Figure 2b). Corresponding 1, 3- and 5-year OS for all four groups are listed in Table 3.
| All | Chemo1 | Local2 | Multimodal3 | Untreated4 | |
| Patients, n (%) | 305 (100) | 76 (25) | 46 (15) | 21 (7) | 162 (53) |
| Overall survival | |||||
| One year | 23% | 45% | 22% | 38% | 12% |
| Three year | 10% | 12% | 10% | 10% | 5% |
| Five year | 8% | 10% | 7% | 5% | 3% |
| Hospitalisation | |||||
| Average number, any cause | 5.0 | 9.0 | 5.3 | 9.1 | 2.5 |
| Total days per patient, median | 22 | 43 | 38 | 49 | 12 |
| (IQR) | (7–46) | (21–65) | (19–54) | (39–77) | (1–27) |
| 1Chemotherapy only. | |||||
| 2Major local treatment (cystectomy or pelvic radiotherapy) only. | |||||
| 3Chemotherapy in combination with any local treatment (cystectomy, pelvic radiotherapy, transurethral resection of bladder tumour (TURBT)) or a combination of local treatments. | |||||
| 4No chemotherapy and no local treatments except TURBT only. | |||||
Hospitalisation
In 2008–2016, there were 5,635 registered contacts with the hospital, of which 1,498 (27%) were inpatient contacts. The average number of hospitalisations of any cause (pre-planned and emergency admissions included) for all patients was 5.0, ranged from 2.5 (untreated) to 9.1 hospitalisations (multimodal) (Table 3). For patients in the chemo group, the average number of hospitalisations was 5 when chemotherapy delivery hospitalisations were excluded. Median total days of hospitalisation per patient were 22 days (Table 3). Compared to the untreated group (median 12 days, IQR: 1–27), the median total days of hospitalisation per patient was three to four times higher amongst patients in the local (38 days, IQR: 19–54), multimodal (49 days, IQR: 39–77) and chemo (43 days, IQR: 21–65) groups.
Discussion
In this nationwide population-based study of patients with primary mBC, approximately one-third of the patients started chemotherapy within 150 days after diagnosis. During the first 150 days after BC diagnosis, few patients were treated with initial major local tumour procedures, and more than 50% of the patients were not treated with any anti-cancer treatment. Median OS was 7 months longer for patients treated with chemotherapy compared to patients in the untreated group. Patients in the chemotherapy group had almost four times more days in hospital compared to the patients in the untreated group.
Table 4 compares our results with those from relevant published data. Median OS for our patients in the chemo group was inferior to the results from two clinical trials (9.8 months vs. 12–14 months), which investigated the effect of gemcitabine and cisplatin on OS [3, 24]. Median age of patients in our study was similar to the age of patients included in these two trials. However, 30% of patients included in our study were diagnosed with non-UC histologies, whereas all patients in the displayed trials had UC [25]. Moreover, the prevalence of visceral metastases, a poor prognosis feature [5, 26], was largest in our cohort. Other possible prognostic differences between our and the displayed trial populations are the inclusion of patients with locally advanced disease (T4bN0M0) and secondary metastatic disease. Furthermore, unlike our study, all patients in these trials were treated with cisplatin, whereas we also included patients treated with carboplatin-based chemotherapy. In accordance with this, median OS in our study was closer to median OS (9.3 months) in a clinical trial with gemcitabine and carboplatin (not listed in Table 4) [8].
| Our study | von der Maase [3] (2000) | Bellmunt [24] (2012) | Flannery [18] (2018) | Richters [19] (2020) | Reesink [27] (2020) | Omland [28] (2021) | |
| Type of study | Observational | Clinical trial | Clinical trial | Observational | Observational | Observational | Observational |
| Data | National cancer registry | SEER | National Cancer registry | Multi-centre | Nationwide multicentre | ||
| Country | Norway | Multinational | Multinational | USA | The Netherlands | The Netherlands | Denmark |
| Period | 2008–2016 | 1996–1998 | 2001–2004 | 2007–2011 | 2016–2017 | 2008–2016 | 2010–2016 |
| Primary metastatic | Yes | No | No | Yes | Yes | Yes | No |
| Patients, n | 305 | 405 | 626 | 1215 | 636 | 64 | 952 |
| Cancer | BC | UTC | UTC | BC | BC | BC | UTC |
| Systemic chemotherapy | Unspecified | Gemcitabine and cisplatin | Gemcitabine and cisplatin | Unspecified | Platinum based | Platinum based | Platinum-based or gemcitabine |
| Patients, n (%) | 76 (25) | 203 (100) | 314 | 411 (34) | 198 (31) | 24 (38) | 952 (100) |
| Age (median) | 63 | 63 | 61 | 75 | 70 (carboplatin) 66 (cisplatin) |
65 | 69 |
| Sex (%male) | 69 | 79 | 81 | 70 | 88 | 72 | |
| Urothelial carcinoma (%) | 71 | 100 | 100 | - | 85 | 100 | 92 |
| Visceral metastases (%) | 80 | 69 | 49 | 61 | 69 | 69 | |
| Median overall survival (months) | 9.8 | 14 | 12.7 | 13.2 | 11.1 (carbo-platin), 12.9 (cisplatin) | 12.6 | 11.7 |
| Untreated | |||||||
| Patients, n (%) | 162 (53%) | 804 (66%) | 415 (65%) | 40 (62%) | |||
| Age(median) | 79 | 80 | 76 | 78 | |||
| Sex (% male) | 66 | 58 | 67 | 72 | |||
| Urothelial carcinoma (%) | 68 | 77 | 100 | ||||
| Visceral metastases (%) | 91 | 69 | 81 | 100 | |||
| Median overall survival (months) | 2.3 | 3.2 | 2.5 | 2.0 |
Compared to other real-world studies, a lower proportion of our patients received chemotherapy (Table 4) [18, 19, 27]. However, if we include patients treated with a multimodal approach, which included chemotherapy, approximately 31% of patients received initial chemotherapy, similar to the registry-based studies by Flannery et al. [18] and Richters et al. [19]. Compared to our study, the patients of these registry- [18, 19] and multicentre-based [27, 28] studies were older. However, fewer patients had visceral metastases and non-UC histology. This might explain the slightly better median OS (11–13 months) in these studies compared to our results (9.8 months) [18, 19, 27, 28]. Finally, a survival difference may also be explained by other for us unknown adverse risk factors in our population (performance status, comorbidities and renal function), which may have impacted choice of treatment and prognosis [7, 26].
Patients treated with chemotherapy were frequently hospitalised with an average of 9 all-cause hospitalisations during follow-up. In comparison, Flannery et al. [18] reported an average of 5.2 all-cause hospitalisations in the same patient group. The difference between our population and the population in Flannery et al. [18] may be related to differences in the setting of care for chemotherapy administration, with possibly more patients in Norway receiving chemotherapy as inpatients as indicated by the reduction of median hospitalisation from 9 to 5 when chemotherapy delivery hospitalisations were excluded. The difference in average number of hospitalisations and median hospitalisation days in patients receiving treatment versus those receiving no treatment can in part be explained by the fact that ‘hospitalisation days’ also included pre-planned treatment delivery days (chemotherapy, cystectomy and RT). For patients who did not receive any anti-cancer treatment, the priority was most likely to discharge to either home or community whenever possible, resulting in shorter hospital stay. Some of these patients probably did not require readmission since such patients usually are cared for at home or closer to home with the support of palliative care and primary care teams. In addition, the short survival of the untreated patients results in fewer and shorter hospitalisations.
In accordance with comparable studies, a large proportion of patients did not receive chemotherapy (Table 4) [18, 19, 27]. Similar to these studies, the untreated patients were older, more frequently female and more had visceral metastases compared to patients treated with chemotherapy. Median OS in our study was comparable to the survival reported in these studies.
This study is limited by its descriptive design, making intergroup comparisons of outcomes difficult due to many unknown confounders. Important patient-related factors that may influence treatment and survival such as performance status, comorbidities, frailty score and renal function were unavailable [7, 26]. Because we lacked the relevant information, we could not report on important tumour-related factors such as clinical T-category or provide the number and exact location of extra pelvic lymph node metastases or other distant metastases. Due to the CRN’s data collection practices, metastatic disease was registered as ‘metastases present at diagnosis’ if metastases were detected within 150 days from diagnosis. If metastases occurred within 150 days despite initial local treatment (e.g. cystectomy or PRT), these were still coded as metastases at diagnosis despite patients being treated with curative intent. Random errors of registration cannot be ruled out. Of the treatment-related factors, we lacked detailed information on the indication for chemotherapy (e.g. neoadjuvant vs. palliative) as well as the dosage of specific drugs/drug combinations. The focus of this paper was on the description of the initial treatment of BC patients; therefore, any subsequent systemic treatment lines beyond the first treatment received were not considered in this study.
The described burden of hospitalisation is applicable to routine practice in Norway and may not be generalisable to other geographical areas due to differences in the organisation of national healthcare services. However, in this first Norwegian study of unselected patients with primary mBC, we provide an overview over the real-world initial treatment and prognosis before the introduction of novel agents into routine clinical practice. Our study is therefore a baseline representation of patients with this tumour type and highlights the challenges faced with delivery of platinum-based chemotherapy in a frailer and comorbid patient group. Our real-world data set is an important benchmark against which future analyses of survival outcomes of newer therapies (including immunotherapy and novel agents) and optimising care pathways can be measured.
Conclusion
In this pre-immunotherapy population-based study, the majority of Norwegian patients with primary mBC did not receive any kind of anti-cancer treatment and had a dismal prognosis. Compared to relevant clinical trials, such patients treated with chemotherapy had inferior OS. Further studies should evaluate whether the introduction of novel therapies, such as less toxic immunotherapy, enables anti-cancer treatment of a larger proportion of patients with primary mBC and results in a more favourable survival and a reduced burden of hospitalisation.
Authors’ contributions
CTM: Project development, Data management and analysis, Manuscript writing; GT: Project development, Manuscript editing; AB: Data collection, Manuscript editing; VB: Project development, Manuscript editing; SDF: Project development, Manuscript writing; BKA: Protocol and project development, Data collection, management and analysis, Manuscript writing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approved by the Regional Committee for Medical and Health Research Ethics, Southeast Norway. Approval number: 2016/2286/REK sør-øst A. The study is also approved by the CRN and the NPR. All data management and analyses were conducted according to the current legislation and regulation of privacy, without any possibilities for individual identification. The requirement for informed consent was waived by the Regional Committee for Medical and Health Research Ethics, Southeast Norway.
Availability of data and materials
The data that support the findings of this study are available from the CRN. Restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the CRN.
Biography of authors
Christina Tanem Møller, MD, Oncologist and PhD student at the Cancer Registry of Norway, Norway. https://orcid.org/0000-0002-1916-4094
Gunnar Tafjord, MD, Oncologist at Oslo University Hospital, Norway. https://orcid.org/0000-0002-6614-5048
Augun Blindheim, MD, Urologist at St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway and Associate Professor at the Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway. https://orcid.org/0000-0002-4258-2841
Viktor Berge, MD, Urologist at Oslo University Hospital, Norway and Professor at the Faculty of Medicine, University of Oslo, Norway. https://orcid.org/0000-0003-3349-5794
Sophie D. Fosså, MD, Oncologist and Professor Emerita at the Faculty of Medicine, University of Oslo, Norway. https://orcid.org/0000-0002-3729-2217
Bettina Kulle Andreassen, Statistician/Epidemiologist, PhD and senior researcher at the Cancer Registry of Norway. https://orcid.org/0000-0001-5087-2913
References
- [1] Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(3):244–58. https://doi.org/10.1016/j.annonc.2021.11.012
- [2] Witjes JA, Cathomas R, Compérat E, et al. The EAU Guidelines on Muscle-invasive and Metastatic bladder cancer 2022. [Accessed Aug, 2022] Available from: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/
- [3] von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77. https://doi.org/10.1200/JCO.2000.18.17.3068
- [4] Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15(7):2564–9. https://doi.org/10.1200/JCO.1997.15.7.2564
- [5] von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. https://doi.org/10.1200/JCO.2005.07.757
- [6] Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432–8. https://doi.org/10.1200/JCO.2011.34.8433
- [7] Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107(3): 506–13. https://doi.org/10.1002/cncr.22031
- [8] De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer ‘unfit’ for cisplatin-based chemotherapy: phase II – results of EORTC study 30986. J Clin Oncol. 2009;27(33):5634–9. https://doi.org/10.1200/JCO.2008.21.4924
- [9] Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. https://doi.org/10.1056/NEJMoa1613683
- [10] Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. https://doi.org/10.1016/S0140-6736(16)00561-4
- [11] Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3): 312–22. https://doi.org/10.1016/S1470-2045(17)30065-7
- [12] Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–30. https://doi.org/10.1056/NEJMoa2002788
- [13] Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–600. https://doi.org/10.1200/JCO.19.01140
- [14] Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–35. https://doi.org/10.1056/NEJMoa2035807
- [15] Cancer in Norway 2021 – cancer incidence, mortality, survival and prevalence in Norway [Internet]. Oslo: Cancer Registry of Norway; 2022. [Cited Aug, 2022] Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2021/cin_report.pdf [cited 2022].
- [16] Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølgning av blærekreft Oslo: Helsedirektoratet; 2013. Available from: https://blaerekreft.no/wp-content/uploads/Nasjonalt-handlingsprogram-bl%C3%A6rekreft.pdf [cited 3 August 2021].
- [17] Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av blære- og urotelkreft 2021. [Cited Aug, 2022] Available from: https://www.helsedirektoratet.no/retningslinjer/blaerekreft-handlingsprogram/Bl%C3%A6re-%20og%20urotelkreft%20%E2%80%93%20Nasjonalt%20handlingsprogram%20med%20retningslinjer%20for%20diagnostikk,%20behandling%20og%20oppf%C3%B8lging.pdf/_/attachment/inline/4a169897-6aec-49c9-be35-c5b1e14fd7d6:b1dba4fcdeae13b6bb65579bdc1fbafeff4b69eb/Nasjonalt%20handlingsprogram%20med%20retningslinjer%20for%20diagnostikk,%20behandling%20og%20oppf%C3%B8lging%20av%20bl%C3%A6re-%20og%20urotelkreft.pdf.
- [18] Flannery K, Cao X, He J, et al. Survival rates and health care costs for patients with advanced bladder cancer treated and untreated with chemotherapy. Clin Genitourin Cancer. 2018;16(4):e909–17. https://doi.org/10.1016/j.clgc.2018.03.002
- [19] Richters A, Mehra N, Meijer RP, et al. Utilization of systemic treatment for metastatic bladder cancer in everyday practice: results of a nation-wide population-based cohort study. Cancer Treat Res Commun. 2020;25:100266. https://doi.org/10.1016/j.ctarc.2020.100266
- [20] Galsky MD, Pal SK, Lin SW, et al. Real-world effectiveness of chemotherapy in elderly patients with metastatic bladder cancer in the united states. Bladder Cancer. 2018;4(2):227–38. https://doi.org/10.3233/BLC-170149
- [21] Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–31. https://doi.org/10.1016/j.ejca.2008.10.037
- [22] Bakken IJ, Ariansen AMS, Knudsen GP, et al. The Norwegian Patient Registry and the Norwegian Registry for Primary Health Care: research potential of two nationwide health-care registries. Scand J Public Health. 2019;48(1):49–55. https://doi.org/10.1177/1403494819859737
- [23] Nasjonal tjeneste for validering og dekningsgradsanalyser – Årsrapporter: Helsedirektoratet. [Cited Sep, 2022] Available from: https://www.helsedirektoratet.no/rapporter/nasjonal-tjeneste-for-validering-og-dekningsgradsanalyser-og-arsrapport.
- [24] Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30(10):1107–13. https://doi.org/10.1200/JCO.2011.38.6979
- [25] Veskimäe E, Espinos EL, Bruins HM, et al. What is the prognostic and clinical importance of urothelial and nonurothelial histological variants of bladder cancer in predicting oncological outcomes in patients with muscle-invasive and metastatic bladder cancer? A European association of urology muscle invasive and metastatic bladder cancer guidelines panel systematic review. Eur Urol Oncolgy. 2019;2(6):625–42. https://doi.org/10.1016/j.euo.2019.09.003
- [26] Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–81. https://doi.org/10.1200/JCO.1999.17.10.3173
- [27] Reesink DJ, van de Garde EMW, Peters BJM, et al. Treatment patterns and clinical outcomes of chemotherapy treatment in patients with muscle-invasive or metastatic bladder cancer in the Netherlands. Sci Rep. 2020;10(1):15822. https://doi.org/10.1038/s41598-020-72820-y
- [28] Omland LH, Lindberg H, Carus A, et al. Real-world treatment patterns and overall survival in locally advanced and metastatic urothelial tract cancer patients treated with chemotherapy in Denmark in the preimmunotherapy era: a nationwide, population-based study. Eur Urol Open Sci. 2021;24:1–8. https://doi.org/10.1016/j.euros.2020.12.002