ORIGINAL RESEARCH ARTICLE
A randomised study of TURP after intraprostatic injection of mepicacaine/adrenaline versus regular TURP in patients with LUTS/BPO
Fredrik Stenmarka,b, Lars Brudinc,d, Olof Gunnarssonb, Henrik Kjölhedea,e, Edvard Lekåsf, Ralph Peekera,e, Marianne Månssona, Jonas Richthoffg and Johan Strannea,e
aDepartment of Urology, Institute of Clinical Science, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; bDepartment of Urology, Kalmar County Hospital, Kalmar, Sweden; cDepartment of Medicine and Health Sciences, University Hospital in Linköping, Linköping, Sweden; dDepartment of Clinical Physiology, Kalmar County Hospital, Kalmar, Sweden, eDepartment of Urology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden, fDepartment of Urology, Växjö County Hospital, Växjö, Sweden; gDepartment of Urology, Ljungby County Hospital, Ljungby, Sweden
ABSTRACT
Introduction: During transurethral resection of the prostate (TURP), the most established surgical treatment of lower urinary tract symptoms (LUTS) due to benign prostatic obstruction (BPO), the prostate can bleed profusely, bringing about anaemia and compromised oxygen delivery to the entire body.
Objective: The primary objective of this study was to assess the efficacy of mepivacaine and adrenaline (MA) injected into the prostate on bleeding. The primary endpoint was to measure blood loss per resected weight of prostate tissue.
Material and methods: This randomised controlled trial evaluated 81 patients with LUTS/BPO. Patients were randomly allocated to regular TURP or TURP with intraprostatic injections of MA.
Results: On univariable analyses there was a significant difference in resection weight in favour of the experimental group, not reflected by a statistically significant difference in the other studied outcome parameters. Nevertheless, in multivariable analyses, blood loss per resection weight, which was the primary outcome, showed a significant decrease in favour of the experimental group. Clavien–Dindo complication classification showed three men with a grade I complication and two men with grade II.
Conclusions: The results obtained in this study showed that it is beneficial to apply intraprostatic injections of MA in immediate conjunction with TURP, in terms of blood loss per resected gram. The study is, however, small and corroboration of our results in more extensive prospective studies may therefore be warranted before embarking upon this technique.
KEYWORDS: TURP; intraprostatic injection; mepivacaine/adrenaline
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 46–51. https://doi.org/10.2340/sju.v58.7798.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 23 January 2023 Accepted: 5 June 2023 Published: 24 August 2023
CONTACT Johan Stranne johan.stranne@vgregion.se Department of Urology, Sahlgrenska University Hospital, SE-413 45 Gothenburg, Sweden
Introduction
Transurethral resection of the prostate (TURP) is the most established surgical method in patients with lower urinary tract symptoms (LUTS) due to benign prostatic obstruction (BPO). Its place as a reference method and the gold standard is in many ways unquestioned. However, there are alternatives that may be amenable, especially in men having prostates <30 mL and >80–100 mL [1–3]. Furthermore, the prostate can bleed profusely during and after a TURP, bringing about anaemia and compromised oxygen delivery to the entire body, in some cases necessitating blood transfusion [4, 5].
Moreover, excessive bleeding can severely disturb the vision during surgery, but bleeding is by no means the only negative consequence of open standing intraprostatic vessels. The opening of intraprostatic veins during a TURP also entails risk of resorption of irrigation fluid which, in turn, can lead to hyponatremia, a condition that, in its most severe form can bring about life-threatening cerebral oedema [6, 7]. There are two different techniques of TURP in use, the more common monopolar and the more recently introduced bipolar technique. These techniques have been compared in randomized trials, with respect to bleeding, fluid absorption and other complications, with inconsistent results [8–10]. Reducing intraprostatic blood flow during surgical procedures has been addressed previously. For example, intraprostatic injections of mepivacaine and adrenaline (MA) immediately prior to transurethral microwave thermotherapy (TUMT) have been shown to significantly decrease blood flow during treatment, considerably shortening treatment time as well as energy consumption [11–14].
The hypothesis of this study was therefore that injection of MA in the prostate prior to TURP would decrease bleeding and thereby facilitate a more thorough resection of prostate tissue. The primary endpoint was to measure blood loss (mL) per resected weight of prostate tissue (g) during TURP. Secondary endpoints were resection weight, perioperative bleeding, change in postoperative haemoglobin level at day one, transfusion and complication rate.
Material and methods
Patients
This randomised controlled trial, ethical approval (dnr M238-08), included 100 patients with LUTS, which was judged secondary to BPO, where TURP was deemed the preferred treatment option. Evaluation and surgery were performed at three hospitals in Sweden. After inclusion, patients were randomly allocated 1:1 to one of two groups: group A (control group): regular TURP (n = 50) and group B (interventional group): TURP with intraprostatic injections of MA (n = 50). Randomization was performed by the use of envelopes taken from a box.
The inclusion criteria were: patients with verified prostate enlargement deemed suitable for TURP, measured by transrectal ultrasound (TRUS) >30 mL (TRUS: Flex Focus, BK Medical, Herlev, Denmark), symptoms corresponding to >12 points according to the international prostate symptom score (IPSS) and finally a maximum free flow rate (Qmax) of <13 mL/s. The exclusion criteria were: known intolerance to mepivacaine or adrenaline or patients unfit to tolerate TURP operation (e.g. severe bleeding disorders or high ASA score). Before treatment, the following baseline parameters were recorded: age, height, weight, prostate volume, IPSS, including the quality-of-life question (QoL), peak urinary flow (Qmax), haemoglobin, comorbidity and current LUTS and non-steroidal anti-inflammatory drugs (NSAID) medications. In case of treatment with anticoagulants such medication was discontinued 3–5 days prior to surgery with the use of bridging low-molecular-weight heparins (LMWHs). LMWH was also generously employed as thrombosis prophylaxis in Sweden during the time of the study.
Procedure
Surgery was performed according to each surgeon’s preference. All patients had spinal or general anaesthesia, depending on the hospital’s routine. Perioperative irrigation fluid was mannitol, with ethanol for the intermittent tracing of ethanol in the breathing air to detect irrigation fluid absorption. Postoperatively all patients had a three-way catheter for irrigation, forced by diuretic medication and bladder irrigation on demand. MA was given to the patients in group B, administered by the transurethral route using the previously described Schelin Catheter (Schelin Catheter™, ProstaLund AB, Lund, Sweden) [12]. This catheter device harbours a balloon at the tip, making it possible to anchor it at the bladder neck. A cannula (1.2 mm in diameter) for injection is oriented separately in the catheter wall. It exits the catheter with an angle of 30°, at the level of the prostatic urethra. The cannula penetrates the prostate to a depth of 45 mm, reaching the base of the prostate irrespective of prostate size, while its deep position always correlates to the bladder neck. This device enables medication of the prostate blindly by the transurethral route. The catheterisation and the injection technique followed the previously described procedure [15]. In brief, the catheter was inserted immediately prior to surgery, and the balloon was filled with 15 mL of sterile water. The catheter was thereafter rotated to the 8 o’clock position (on the patient’s right side) and retracted until anchored at the bladder neck. The injection cannula was then introduced to its full length to hit the base area of the prostate. Prostate injections were performed by the same injection technique at four sites; 5 mL of 0.5% MA was injected intermittently, 1 mL at a time when the cannula was fully introduced in the deep position. After that, another 5 mL was administered as an intermittent stepwise infiltration, 1 mL at a time, while the cannula is retracted. Each injection was preceded by aspiration to avoid intravascular injection. Then the cannula was fully retracted, and the catheter was reintroduced deep into the bladder. After rotation of the catheter to the 11 o’clock position, it was retracted down to the bladder neck once again until the balloon anchored in the bladder neck. The injection procedure was then repeated at the 11 o’clock position using the same technique. After completing these two injections, the catheter was removed, and the resection of the right prostate lobe was completed. The Schelin Catheter was then reinserted, the balloon was filled with 40 mL of sterile water to avoid retraction of the catheter balloon down into the resection cavity, and by the same technique, additional intraprostatic infiltrations of 2 × 10 mL of 0.5% MA was performed in the 4 and 1 o’clock positions, upon which resection of the left prostate lobe was completed. Perioperative bleeding was measured by photometric technique, according to hospital routine. Other parameters recorded postoperatively were operative time, resection weight, irrigation time, and complications within 30 days after surgery was described and classified according to a modified Clavien–Dindo system as recently described [16, 17].
Statistics
Descriptive statistics of patients’ characteristics, bleeding and the endpoints were presented with medians and ranges for continuous variables and frequencies and proportions for categorical variables. The primary and secondary endpoints were compared between the treatment groups using univariable (unadjusted) and multivariable (adjusted) linear regression. The adjustment variables were age, 5-alpha reductase inhibitors (5ARIs), NSAID, and LMWHs. The endpoints bleeding per resected weight and resection weight were both logarithmically transformed before the regression analysis due to their skewed nature in order to fulfil the model requirement of normally distributed residuals. Hence, the results are interpreted in terms of fold change. Haemoglobin was analysed as a change from baseline.
P-values below 0.05 were considered statistically significant and no adjustment for multiple comparisons was performed. The statistical analyses were carried out using R Statistical Software (Version 4.2.3) and Statistica (Version 13.5.0.17).
Results
Patient characteristics
A total of 81 men were eligible for evaluation, Figure 1, and baseline data are presented in Table 1. The patients randomised to MA were in median 5 years older. The most frequent concurrent conditions were high blood pressure, evident in 34 men (42%) and hyperlipidaemia, in 17 men (21%).
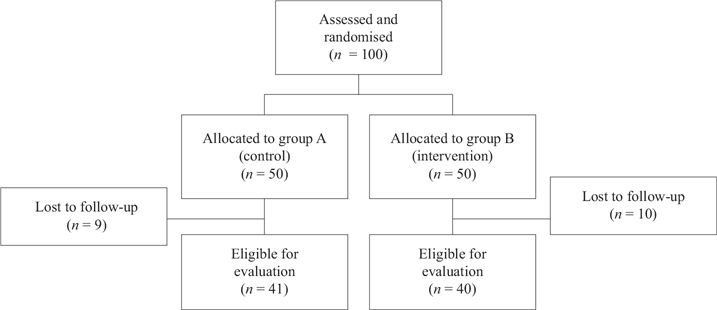
Figure 1. Flow chart regarding enrolment, assessment, randomisation, allocation, follow-up, and analysis. A total of 19 patients were lost to follow-up due to administrative reasons, all from one hospital, equally distributed between group A and B.
Furthermore, 14 men (17%) had cardiovascular diseases such as ischemic heart disease, heart failure or atrial fibrillation. Diabetes mellitus was noticed in 10 men (12%), and pulmonary disease such as bronchial asthma and chronic obstructive pulmonary disease was found in seven men (9%).
Finally, a total of four men (5%) had a medical history of stroke. Regarding medication for LUTS, 5ARIs were used by 14 men (17%), and 26 men (32%) used other medications, for example, alpha1-adrenoceptor antagonists or muscarinic receptor antagonists. A total of 26 men (32%) used NSAIDs, and 39 men (48%) were prescribed LMWHs in connection with surgery.
Complications
A total of five men suffered adverse events occurring in direct association with surgery or hospital stay (4 in the TURP-only group and 2 in the TURP + MA group). There were no further complications after discharge from the hospital, up to 30 days after surgery. No patients needed a blood transfusion. One patient (in the TURP + MA group) had an episode of hypertension and bradycardia during surgery that resolved spontaneously. The remaining AEs were in the TURP-only group, where two patients resorbed minor volumes of irrigation fluid during surgery but did not develop a TUR syndrome. One of these men was also treated with antibiotics due to a urinary tract infection. Another patient was also treated with antibiotics due to a urinary tract infection, with no other adverse events. Finally, one man developed pancreatitis, and also needed prolonged bladder irrigation (>48 h), which extended his hospital stay. These complications were also categorised according to a modified Clavien–Dindo classification of adverse events. In summary, three men suffered from grade I complications and two men from grade II complications.
Main findings on studied parameters
The primary and secondary endpoints are described in Table 2 and the results from both unadjusted and adjusted linear regressions are presented in Table 3. The primary endpoint, bleeding per resection weight, was 6.6 and 4.8 mL/g in the TURP only and TURP + MA groups, respectively. This difference was not statistically significant in the unadjusted analysis (fold change: 0.71% (95% CI: 0.47 to 1.10) P = 0.122). However, in the adjusted regression analysis, MA led to a statistically significant lower bleeding per resection weight by 40% (fold change: 0.60 (95% CI: 0.38 to 0.95) P = 0.030). In contrast, the secondary endpoint total resection weight had a statistically significant increase in the unadjusted analysis (fold change: 1.25 (95% CI 1.01 to 1.25) P = 0.037), but in the adjusted analysis the P-value was just above the significance level despite similar point estimates and a P-value close to the significance level (fold change: 1.23 (95% CI 0.99 to 1.55) P = 0.067). There were no statistically significant differences in change of haemoglobin level or complication rates (Table 3).
| Primary endpoint | Univariable regression analyses | Multivariable regression analyses | ||
| TURP + MA vs. TURP Outcome (95% CI) | P | TURP + MA vs. TURP Outcome (95% CI) | P | |
| Bleeding/resected weight (ml/g)a | 0.71a (0.47 to 1.10) | 0.122 | 0.60a (0.38 to 0.95) | 0.030 |
| Secondary endpoints | ||||
| Perioperative bleeding (mL)a | 0.87a (0.53 to 1.44) | 0.581 | 0.73a (0.42 to 1.25) | 0.247 |
| Resection weight (g)a | 1.25a (1.01 to 1.55) | 0.037 | 1.23a (0.99 to 1.55) | 0.067 |
| Change (from baseline) in haemoglobin level at day one (g/L)b | 4.79b (0.23 to 99.0) | 0.306 | 6.56b (0.25 to 171.7) | 0.254 |
| Complication ratec | 0.51c (0.07 to 2.82) | 0.460 | 0.36c (0.03 to 2.82) | 0.349 |
| aLinear regression/outcome = fold change; bLinear regression/outcome = change; cLogistic regression/outcome = odds ratio) of primary and secondary endpoints. The multivariable regression analyses were adjusted for age, 5ARI, NSAID and LMWH. CI = Confidence interval; TURP = Trans Urethral Resection of Prostate; MA = Mepivacaine adrenaline. | ||||
Discussion
In this randomised controlled study intraprostatic injections of MA before resection decrease the blood loss per resected gram of prostate during a TURP. We could not show a corresponding significant change in any of the secondary endpoints, including total resection weight.
Throughout the years, there have been several efforts to facilitate TURP. In order to diminish prostate size and also decrease perioperative bleeding the use of 5ARIs have been proposed [18, 19]. There are a few reports preceding the present one on injection therapy into the prostate to achieve these goals. In a study by Lira-Dale et al. from 2012 they concluded that intraprostatic injections of adrenaline reduce mean blood loss during TURP by 62% [20]. Furthermore, they did not find that injections of adrenaline increased prostate resection weight. Our results regarding bleeding and prostate resection weight are in line with these results. In that double-blind study, a total of 13 men were randomised to injections with adrenaline and 10 men to injections with saline. Mean blood loss in the group who received adrenaline was 127 mL versus 337 mL in the saline group and there were no statistically significant differences in mean resection time between the two groups. The adrenaline was injected into the prostate via a cannula inserted in a cystoscope, comparable to the technique in our study. However, the two-step injection procedure, one side at a time, applied in the present study, was chosen to optimise the astringent effect from adrenaline by avoiding wash-out on the left side while operating the right side, a notion that has previously been brought forward by Schelin et al. [15]. In that study, it was shown that intraprostatic injections could reduce perioperative blood loss, and a total of 11 men were given MA in the prostate during TURP. Total blood loss, perioperative bleeding, operative time and resected volume were compared with 30 patients who previously had undergone TURP. It was concluded that perioperative bleeding was significantly reduced compared with the control group [15]. However, the bleeding in the control group of both these studies was considerably higher than in our study.
In the present study, severe complications were scarce and numerically even lower in the intervention groups and no patient, regardless of group, required a blood transfusion. Our findings, with low risk of perioperative bleeding and complications during TURP, are at variance with previously reported results. Reich et al. reported, in a study of 10,654 men, a transfusion rate of 2.9% and Cornu et al., in a meta-analysis of 69 RCTs and 8,517 patients, a transfusion rate of 2%, as well as clot retention in 4.9% [4, 5]. We believe that the positive results in our study, together with the low rate of complications, further underscore the importance of our report.
An apparent strength of our study is the prospective randomised design. On the downside is the relatively low number of evaluable patients. Furthermore, it would have been valuable to, in addition to describing concurrent diseases, define comorbidity and frailty of the patients according to a well-defined scale e.g. the ASA or ECOG classification. Some of the used medications may affect bleeding parameters, such as 5ARIs, NSAIDs, and LMWHs. Such medications should, preferably, have been handled more uniformly within the study groups. However, the randomised design, a similar distribution of such medication between the two groups, would indicate that this should not be a problem in our study. Another potential weakness of our study was differences in the time from injection of MA to resection, as wash-out from the prostatic gland occurs rapidly, and resection should start immediately after injection, with minimum delay. However, this mimics the ‘normal’ clinical situation and could therefore also be argued to strengthen our results.
Conclusion
The results obtained in the present study show that intraprostatic injections of MA in immediate conjunction with TURP is beneficial and decreases the blood loss per resected gram during the procedure. The study is, however, small and corroboration of our results in more extensive prospective studies may therefore be warranted before embarking upon this technique in clinical practice full out.
Acknowledgement
We gratefully acknowledge Dr Sonny Schelin for irreplaceable support, knowledge, and experience when the study design was discussed. We also sincerely thank him for conducting the pilot study that laid the foundation for this present study.
References
- [1] Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612–19. https://doi.org/10.1016/j.juro.2018.05.048
- [2] Parsons JK, Dahm P, Kohler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799–804. https://doi.org/10.1097/JU.0000000000001298
- [3] Cornu J-NL, Gravas S, Gacci M, Hashim H, Herrmann TRW, Netsch C, et al. Management of non-neurogenic male LUTS. 2020. Available from: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts [cited 27 March 2020].
- [4] Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol. 2008;180(1):246–9. https://doi.org/10.1016/j.juro.2008.03.058
- [5] Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Gilling P, Gratzke C, et al. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol. 2015;67(6):1066–96. https://doi.org/10.1016/j.eururo.2014.06.017
- [6] Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP) – incidence, management, and prevention. Eur Urol. 2006;50(5):969–79; discussion 980. https://doi.org/10.1016/j.eururo.2005.12.042
- [7] Hawary A, Mukhtar K, Sinclair A, Pearce I. Transurethral resection of the prostate syndrome: almost gone but not forgotten. J Endourol. 2009;23(12):2013–20. https://doi.org/10.1089/end.2009.0129
- [8] Stucki P, Marini L, Mattei A, Xafis K, Boldini M, Danuser H. Bipolar versus monopolar transurethral resection of the prostate: a prospective randomized trial focusing on bleeding complications. J Urol. 2015;193(4):1371–5. https://doi.org/10.1016/j.juro.2014.08.137
- [9] Fagerstrom T, Nyman CR, Hahn RG. Bipolar transurethral resection of the prostate causes less bleeding than the monopolar technique: a single-centre randomized trial of 202 patients. BJU Int. 2010;105(11):1560–4. https://doi.org/10.1111/j.1464-410X.2009.09052.x
- [10] Fagerstrom T, Nyman CR, Hahn RG. Complications and clinical outcome 18 months after bipolar and monopolar transurethral resection of the prostate. J Endourol. 2011;25(6):1043–9. https://doi.org/10.1089/end.2010.0714
- [11] Schelin S, Claezon A, Sundin A, Wagrell L. Effects of intraprostatic and periprostatic injections of mepivacaine epinephrine on intraprostatic blood flow during transurethral microwave thermotherapy: correlation with [15O]H2O-PET. J Endourol. 2004;18(10):965–70. https://doi.org/10.1089/end.2004.18.965
- [12] Schelin S. Mediating transurethral microwave thermotherapy by intraprostatic and periprostatic injections of mepivacaine epinephrine: effects on treatment time, energy consumption, and patient comfort. J Endourol. 2002;16(2):117–21. https://doi.org/10.1089/089277902753619645
- [13] Knutson T, Johansson A, Damber JE, Fall M, Vesely S, Peeker R. Intraurethral prostate injections with mepivacaine epinephrine: effects on patient comfort, treatment time and energy consumption during high-energy transurethral microwave thermotherapy. Scand J Urol Nephrol. 2009;43(4):300–6. https://doi.org/10.1080/00365590903092889
- [14] Stenmark F, Brudin L, Stranne J, Peeker R. High-energy feedback microwave thermotherapy and intraprostatic injections of mepivacaine and adrenaline: an evaluation of calculated cell kill accuracy and responder rate. Scand J Urol. 2014;48(4):374–8. https://doi.org/10.3109/21681805.2013.879921
- [15] Schelin S. Transurethral resection of the prostate after intraprostatic injections of mepivacain epinephrine: a preliminary communication. Scand J Urol Nephrol. 2009;43(1):63–7. https://doi.org/10.1080/00365590802465061
- [16] Sagen E, Namnuan RO, Hedelin H, Nelzén O, Peeker R. The morbidity associated with a TURP procedure in routine clinical practice, as graded by the modified Clavien-Dindo system. Scand J Urol. 2019;53(4):240–5. https://doi.org/10.1080/21681805.2019.1623312
- [17] Mamoulakis C, Efthimiou I, Kazoulis S, Christoulakis I, Sofras F. The modified Clavien classification system: a standardized platform for reporting complications in transurethral resection of the prostate. World J Urol. 2011;29(2):205–10. https://doi.org/10.1007/s00345-010-0566-y
- [18] Crea G, Sanfilippo G, Anastasi G, Magno C, Vizzini C, Inferrera A. Pre-surgical finasteride therapy in patients treated endoscopically for benign prostatic hyperplasia. Urol Int. 2005;74(1):51–3. https://doi.org/10.1159/000082709
- [19] Donohue JF, Sharma H, Abraham R, Natalwala S, Thomas DR, Foster MC. Transurethral prostate resection and bleeding: a randomized, placebo controlled trial of role of finasteride for decreasing operative blood loss. J Urol. 2002;168(5):2024–6. https://doi.org/10.1016/S0022-5347(05)64287-5
- [20] Lira-Dale A, Maldonado-Avila M, Gil-Garcia JF, Mues-Guizar EH, Nerubay-Toiber R, Guzmán-Esquivel J, et al. Effect of intraprostatic epinephrine on intraoperative blood loss reduction during transurethral resection of the prostate. Int Urol Nephrol. 2012;44(2):365–9. https://doi.org/1007/s11255-011-0071-2