ORIGINAL RESEARCH ARTICLE
External beam radiotherapy of prostate cancer with or without high dose-rate brachytherapy: the Norwegian experience with long-term urinary and bowel adverse effects
Trude B. Weddea, Milada C. Smaastuenb, Kari Vatnea, Melanie Birthe Schulz-Jaavalla, Sophie D. Fossåc# and Wolfgang LH. Lillebya
aDepartement of Oncology, Oslo University Hospital, Oslo, Norway; bDepartement of Health Science, Oslo Metropolitan University, Oslo, Norway; cNational Advisory Unit for Late Effects After Cancer Treatment, Oslo University Hospital, The Norwegian Radium Hospital, Oslo, Norway
ABSTRACT
Background: There are few studies utilizing the Expanded Prostate Index Composite questionnaire-26 (EPIC-26) questionnaire to examine the long-term association between Domain Summary Scores (DSSs) and Quality of Life (QoL) after External Beam Radiation Therapy (EBRT, 3DCRT [3D conventional radiotherapy]/IMRT [intensity modulated radiation therapy]) versus EBRT combined with High-Dose-Rate Brachytherapy (BT+, 3DCRT [3D conventional radiotherapy]/IMRT). In this cross-sectional study we compare long-term adverse effects and QoL after BT+ with EBRT.
Methods: Prostate Cancer Survivors who at least 5 years previously, had undergone BT+ at Oslo University Hospital between 2004 and 2010 (n = 259) or EBRT (multicentre cohort) between 2009 and 2010 (n = 99) completed a questionnaire containing EPIC-26, Short Form-12 and questions regarding comorbidity/social status. Results were presented as DSSs and Physical/Mental Composite Scores of QoL (PCS/MCS). Regression analyses explored firstly the associations between treatment modality and DSSs and secondly the impact of DSSs on QoL. We estimated the proportions of patients with big/moderate problems. Clinical relevance was set according to the lowest limit of published Minimal Important Differences. P-values <0.05 were considered statistically significant.
Results: In multivariate analysis, only the urinary incontinence DSS remained statistically (P < 0.05) and clinically significantly greater after BT+ than EBRT (90 vs. 83). The number of men with moderate/big urinary or bowel problems was halved after BT+ (P < 0.05). The number of patients with impaired PCS (score < 45) were lower in the BT+ group than the EBRT group (P = 0.02). Regression analysis showed that decreasing levels of bowel and urinary irritation/obstructive DSSs predicted worsening of PCS (P < 0.001) and MCS (P = 0.007), respectively.
Conclusions: Dose-escalated radiotherapy by BT did not negatively impact long-term adverse effects, substantial problems or QoL compared with EBRT. Future randomised studies using improved EBRT techniques are needed.
KEYWORDS: Brachytherapy; prostate cancer; patient-report outcomes; adverse effects; epic-26; QoL
Citation: Scandinavian Journal of Urology 2023, VOL. 58, 68–75. https://doi.org/10.2340/sju.v58.9571.
Copyright: © 2023 The Author(s). Published by MJS Publishing on behalf of Acta Chirurgica Scandinavica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Received: 12 February 2023; Accepted: 5 June 2023; Published: 30 August 2023
CONTACT Trude Wedde trudewedde@hotmail.com Department of Oncology, Oslo University Hospital, Oslo, Norway
Supplemental data for this article can be accessed online at https://doi.org/10.2340/sju.v58.9571
#Shared senior authorship.
Highlights
- Long-term urinary and bowel patient-reported adverse effects shows more urinary incontinence after external beam radiation therapy (EBRT) than EBRT in combination with high-dose-rate brachytherapy (BT+).
- Lower prevalence of substantial urinary and bowel problems is seen after BT+ compared EBRT.
- Worsening functional bowel and urinary irritative/obstructive symptoms is associated with decreased physical and mental Quality of Life, respectively.
Introduction
Radiotherapy (RT) represents a curative treatment for Prostate Cancer (PCa) and can be delivered as External Beam Radiation Therapy (EBRT) alone or in combination with high-dose-rate brachytherapy (BT) (abbreviated BT+ in this article) [1–4]. BT+ enables the provision of particularly high target doses to the prostate and is associated with favourable survival rates [5, 6].
After RT, late urinary and bowel adverse effects (‘typical AEs’) represent a major concern [7–9] with the risk increasing with higher radiation doses [10]. Importantly, with a post-RT 10-year overall survival of > 75% [5] such typical advers effects (AEs) may affect the Quality of Life (QoL) of prostate cancer survivors (PCaSs) for many years.
Several publications have described patient-reported post-RT typical AEs in PCaSs followed-up for up to 5 years after BT+ [2]. But few reports have assessed post-BT+ toxicity in long-term PCaSs surveyed for up to 10 years. Furthermore, only scarce information is available about the associations between typical post-BT+ AEs and generic QoL.
With this background, the main objective of this cross-sectional cohort study was to describe long-term typical AEs and their associations with QoL in PCaSs who had undergone BT+ more than 5 years ago (‘long-term PCaSs’). The results were compared with corresponding findings observed in long-term PCaSs after EBRT alone.
Methods and materials
Patients and treatments
All patients included in this study were diagnosed with PCa without distant metastasis and were considered to have a life expectancy of at least 10 years. For each patient disease was categorized according to the D’Amico classification [11].
BT+ group
Since 2004, BT+ has been offered as an option for curative treatment at Oslo University Hospital to patients with PCa [6]. These patients were routinely included in our approved BT registry and invited to participate in future research. All patients treated between 2004 and 2011 who had signed consent were included in the study.
Exclusion criteria for BT+ were Prostate Specific Antigen (PSA) levels > 50 ng/mL, prostate volume >60 mL, previous transurethral resection of the prostate, comorbidities hindering general anaesthesia, clinical or radiological tumour stage T3b/T4 and unfavourable anatomical conditions for a successful implantation procedure [6, 12].
RT started with two boosts of high-dose-rate BT (Iridium 192, each of 10 Gy) to the prostate gland 2 weeks apart followed by conformal 3D-EBRT, 2 Gy × 25 to the prostate gland and seminal vesicles. Assuming an α/β ratio = 3, the 2 Gy fraction Equivalent Dose (EQD2) was 102 Gy [6].
EBRT group
From January 2009 to December 2010, PCa patients planned for curative RT were included in the Norwegian multicentre prospective observational study evaluating AEs after EBRT or radical prostatectomy [13]. Nine RT centres participated in this study. This study covers the previous unpublished EBRT-arm of long-term PCaSs from the given study. No specific target radiation dose was prescribed in the study protocol. However, most patients received conformal 3D-EBRT administered in 35–39 fractions of 2 Gy (EQD2 70–78 Gy) to the prostate and seminal vesicles according to guidelines [14]. Ten patients had simultaneous EBRT (50 Gy) to the pelvic lymph nodes. Routine use of IMRT/IGRT was not introduced in Norway until 2012. A 10 mm distance between the posterior target field border and the anterior rectum wall was generally viewed as acceptable with weekly controls of the target field.
Patients from both groups received (neo)-adjuvant Androgen Deprivation Therapy (ADT) for up to 3 years depending on the risk group according to European Association of Urology (EAU) guidelines or by physicians’ decision [1, 14].
The long-term survey
In 2016, surviving men from the two groups were invited to complete a mailed questionnaire consisting of the Norwegian versions of Expanded Prostate Index Composite questionnaire (EPIC-26) and of the Short Form-12 [15, 16]. The survey also assessed the men’s social status (partnership, work, education) and comorbidity at the time of answering.
EPIC-26
The EPIC-26 questionnaire covers five domains: urinary incontinence, urinary irritation/obstruction, bowel, sexual and hormonal/vitality function, with each domain containing 4–6 items. In this study, only the urinary and bowel domains were evaluated. According to published instructions, the item scores were calculated and the Domain Summary Scores (DSSs) were established [17]. Each scale ranged from 0 (worst) to 100 (best) with lower scores indicating worse severity. The EPIC question number 5 assessed overall urinary problems and question number 13 evaluated overall bowel problems. The scores were categorised as none (100), very small (75), small (50), moderate (25) or big (0) problem. In this study moderate or big overall problems were combined (substantial problem).
SF-12
Physical Composite Summary Score (PCS) and Mental Composite Summary Score (MCS) were calculated for each PCaS according to published instructions [18]. The PCS assesses general physical function, pain and general health. The MCS items evaluate vitality, social functioning, emotional and mental health. For the general Norwegian population, the mean score of 50 (range 0–100) and standard deviation (SD) of 10 is considered valid for both PCS and MCS [18]. Following Osoba et al., a decrease of 10% (PCS/MCS score ≤ 45) reflects impaired QoL [19].
Statistical analysis
Continuous variables were reported as means with SD or range. Categorical variables were described as frequencies and percentages. Crude between group differences were assessed with chi-square tests (pair of categorical variables) and t-test (continuous variables). To explore possible associations between treatment modality and levels of DSSs, and between the levels of DSSs and QoL, we fitted two multivariate logistic linear regression models. Only variables that reached P < 0.1 in bivariate analyses were entered into these regression models. The results are expressed as regression coefficients (B) with 95% confidence intervals (CI).
Following Skolarus et al. [20], the clinically relevant differences between the DSSs were evaluated based on the lower limits of Minimal Important Difference (MID) and established by the following MID: urinary incontinence: 6, urinary irritative/obstructive: 5 and bowel: 4.
All analyses were considered exploratory so no correction for multiple testing was done and P-values <0.05 were considered statistically significant.
The analyses were carried out in STATA 15.1 and SPSS 26 [21, 22].
Ethical considerations
The study was approved by the Norwegian data protection agency and the Norwegian National Research Ethics Committees (no. 2015/429 and 2016/100).
Results
PCaSs
In the BT+ group, 259 of 313 PCaSs (83%) completed the questionnaire and 99 of 114 (87%) responded in the EBRT group (Table 1). The mean age at the time of diagnosis was 66 years in the BT+ group and 67 years in the EBRT group. The mean time from treatment start to filling in the questionnaire was 8.6 years (range 5–12) in the BT+ group and 6.7 years (range 5–7) in the EBRT group (Table 1). There were more men with high-risk disease in the BT+ group than the EBRT group (Table 1). Heart disease, hypertension, previous history of depression and diabetes mellitus (type 1 or 2) were more often reported in the EBRT group than the BT+ group (P < 0.1, Suppl. Table 1). All patients in the BT+ group received the target dose of EQD2102 Gy. In the EBRT group, 77% of the PCaSs received a target dose of ≥74 Gy.
| Baseline data | BT+ (n = 259) | EBRT (n = 99) |
| Baseline | ||
| Age at diagnosis, mean (range) | 65.9 (50–80) | 67.0 (48–79) |
| Age at filling in questionnaire, mean (range) | 74.5 (57–86) | 73.9 (54–85) |
| Time to filling out questionnaire in years, mean (range) | 8.6 (5–12) | 6.7 (5–7) |
| PSA at diagnosis, mean (range) | 20.9 (1.0–66.0) | 18.7 (4.2–81.0) |
| Married/living as married | 207 (81%) | 77 (81%) |
| Occupation: paid work | 28 (11%) | 13 (13%) |
| clinical T-stage | ||
| Intraprostatic (T1/T2)‡ | 97 (37%) | 61 (62%) |
| Extraprostatic (T3)‡ | 162 (63%) | 38 (38%) |
| Gleason grade | ||
| ≤7b‡ | 175 (68%) | 71 (72%) |
| 8–10‡ | 70 (27%) | 28 (28%) |
| PSA | ||
| ≤10‡ | 61 (24%) | 44 (44%) |
| 10.1 – 19.9 | 85 (33%) | 27 (27%) |
| ≥20‡ | 113 (44%) | 28 (28%) |
| D’Amico risk group | ||
| Low‡ | 1 (<1%) | 16 (16%) |
| Intermediate‡ | 29 (11%) | 22 (22%) |
| High‡ | 229 (88%) | 61 (62%) |
| Pelvic radiation | 0 (0%) | 11 (11%) |
| Radiotherapy dose (EQD2) | ||
| 70 | 0 (0%) | 22 (22%) |
| 74 | 0 (0%) | 32 (32%) |
| 78 | 0 (0%) | 42 (42%) |
| 102 | 259 (100%) | 0 (0%) |
| Hormone treatment | ||
| None‡ | 1 (<1%) | 17 (17%) |
| Yes‡ | 245 (95%) | 54 (55%) |
| Comorbidity | ||
| Heart disease | 41 (16%) | 23 (25%) |
| High blood pressure | 128 (50%) | 56 (59%) |
| Diabetes (type I and II)‡ | 30 (12%) | 20 (21%) |
| Previous history of depression‡ | 17 (7%) | 13 (14%) |
| ±P < 0.05. BT, brachytherapy; EBRT, External Beam Radiation Therapy; EQD, Equivalent Dose. | ||
EPIC-26
Urinary AEs
The DSS of the Urinary incontinence domain and all domain single item scores were significantly higher after BT+ than after EBRT (Table 2, Figure 1). The intergroup difference exceeded the study’s defined MID and thus were considered clinically relevant. Furthermore, compared with BT+, twice as many men treated with EBRT used at least one pad per day due to urinary incontinence (16% vs 7%, P = 0.03). Neither the single item scores nor the DSS of the urinary irritative/obstructive domain revealed any significant intergroup differences. Frequent urination represented the most severe complaint with a 7-point inter-group difference (BT+: 68 vs. EBRT: 61).
| A: EPIC-26 items | BT+ (n = 259) Mean (SD) | EBRT (n = 99) Mean (SD) | P |
| Urinary incontinence | |||
| Domain summary score≠ | 90.0 (17.8) | 82.9 (23.1) | < 0.001 |
| Urinary leaking (item 23) | 88.7 (27.7) (n = 256) |
80.6 (34.9) (n = 99) |
0.02 |
| How big problem was dripping/leaking urine (item 28) | 88.3 (22.3) (n = 244) |
80.0 (27.7) (n = 92) |
0.002 |
| Urinary control (item 26) | 85.9 (19.7) (n = 257) |
78.4 (22.9) (n = 98) |
0.004 |
| Number of pads (item 27) Patients using at least 1 pad per day |
97.0 (11.75) (n = 254) 19 (7.4%) |
92.3 (19.9) (n = 99) 16 (16.2%) |
0.003 0.03 |
| Urinary irritative/obstructive | |||
| Domain summary score | 84.5 (14.5) | 80.6 (17.6) | 0.09 |
| Pain/burning on urination (item 29) | 97.0 (12.0) (n = 237) |
95.3 (13.5) (n = 90) |
0.31 |
| Bleeding with urination (item 30) | 98.5 (8.6) (n = 234) |
96.1 (14.5) (n = 91) |
0.07 |
| Weak urine stream/incomplete emptying (item 31) | 73.8 (28.1) (n = 243) |
70.0 (31.0) (n = 95) |
0.35 |
| Frequent urination (item 33) | 68.3 (30.3) (n = 249) |
61.1 (36.4) (n = 95) |
0.06 |
| Urinary problem | |||
| How big a problem has urinary function been (item 34)? | 79.9 (26.5) (n = 257) |
75.0 (31.7) (n = 99) |
0.14 |
| Substantial problem (big/moderate) n (%) | 27 (10.5%) (n = 257) |
19 (19.2%) (n = 99) |
0.03 |
| Bowel | |||
| Domain summary score | 88.5 (16.2) | 85.5 (16.7) | 0.15 |
| Urgency to have a bowel movement (item 49) | 80.1 (27.3) (n = 254) |
74.7 (31.6) (n = 97) |
0.12 |
| Increased frequency of bowel movements (item 50) | 85.3 (24.8) (n = 243) |
80.8 (26.8) (n = 90) |
0.16 |
| Losing control of stools (item 52) | 90.7 (21.8) (n = 246) |
90.8 (20.9) (n = 92) |
0.97 |
| Bloody stools (item 53) | 96.2 (14.3) (n = 244) |
94.8 (15.5) (n = 99) |
0.44 |
| Abdominal/pelvic/rectal pain (item 54) | 94.0 (16.7) (n = 243) |
90.5 (19.2) (n = 92) |
0.09 |
| Bowel problem | |||
| How big a problem has bowel habits been (item 55)? | 83.0 (25.1) (n = 258) |
79.4 (28.0) (n = 97) |
0.24 |
| Substantial problem (big/moderate) n (%) | 17 (6.6%) (n = 258) |
14 (14.3%) (N = 98) |
0.02 |
| B: Quality of Life (SF-12) | |||
| Physical composite score | 46.4 (10.5) (n = 224) |
44.0 (10.3) (n = 82) |
0.08 |
| Impaired PCS (score ≤ 45) n (%) | 77 (34.4%) | 40 (48.8%) | 0.02 |
| Mental Composite Score | 53.6 (8.0) (n = 224) |
52.1 (9.6) (n = 82) |
0.16 |
| Impaired MCS (score ≤ 45) n (%) | 37 (16.5%) | 19 (23.2%) | 0.18 |
| Statistical significance p < 0.05 in bold. | |||
| ≠Clinical relevance. EPIC, Expanded Prostate Index Composite questionnaire; PCS, Physical Composite Scores; MCS, Mental Composite Scores; BT, brachytherapy; EBRT, External Beam Radiation Therapy; SF, Short Form. | |||
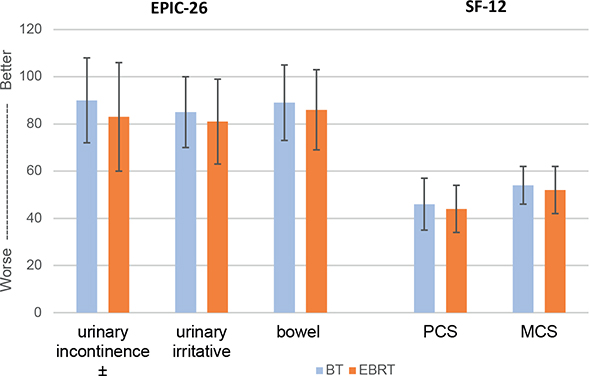
Figure 1. EPIC-26 domain summary scores and physical and mental composite scores (mean ± standard deviation). ± Statistically (P < 0.05) and clinically relevant. EPIC, Expanded Prostate Index Composite questionnaire.
The proportion of men reporting a substantial overall urinary problem was almost doubled in the EBRT group compared with the BT+ group (19% vs. 11%, P = 0.03) (Table 2, Figure 2).
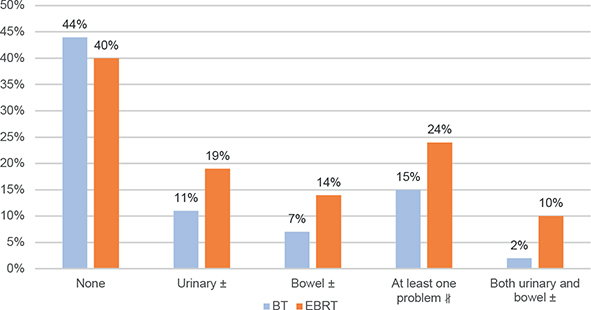
Figure 2. Percentage of patients with substantial urinary and bowel problems. ± P < 0.05, #P = 0.06. BT, brachytherapy; EBRT, External Beam Radiation Therapy.
Bowel AEs
No statistically significant inter-group difference emerged for the bowel DSS (P = 0.15, MID 3), however the single item scores after BT+ were numerically higher than after EBRT (Table 2, Figure 1). Twice as large proportion of men after EBRT than after BT+ reported substantial overall bowel problems (14% vs. 7%, P = 0.02) (Table 2, Figure 2).
Significantly larger proportion of PCaSs reported both urinary and bowel substantial problems in the EBRT group than in the BT+ group (10% vs. 2%, P < 0.05). However, in men reporting no substantial problem, there were no difference between the two groups (Figure 2).
SF12
Despite similar scores for PCS and MCS in the two treatment groups, the proportion of PCaSs with impaired PCS was statistically significantly smaller after BT+ than after EBRT (34% vs 49%, P = 0.02) (Table 2).
Multivariate analyses
Men treated with EBRT had on average almost nine points lower scores for urinary incontinence DSS compared with the BT+ group (B = -8.82; 95% CI [-16.0 to -1.64], P = 0.02) (Tables 3 and 4).
| Variables | Urinary incontinence | Urinary irritation/obstruction | Bowel | ||||||
| Regression coefficient B | 95% CI | P | Regression coefficient B | 95% CI | P | Regression coefficient B | 95% CI | P | |
| Treatment* | –8.82 | –16.0, –1.64 | 0.02 | –0.46 | –6.40, 5.49 | 0.88 | –2.96 | –9.23, 3.31 | 0.35 |
| Risk group | –3.27 | –9.31, 2.78 | 0.29 | –2.26 | –7.37, 2.85 | 0.39 | –0.10 | –5.41, 5.21 | 0.97 |
| Months from treatment to survey | –0.09 | –0.23, 0.05 | 0.20 | –0.02 | –0.13, 0.09 | 0.72 | –0.03 | –0.15, 0.09 | 0.65 |
| Age at the time of diagnosis | –0.21 | –0.60, 0.19 | 0.30 | –0.02 | –0.34, 0.31 | 0.93 | 0.10 | –0.24, 0.44 | 0.57 |
| Heart disease‡ | –3.10 | –9.10, 2.90 | 0.31 | –3.54 | –8.57, 1.48 | 0.17 | –0.18 | –5.41, 5.04 | 0.95 |
| High blood pressure‡ | –4.97 | –9.51, –0.43 | 0.03 | –3.07 | –6.81, 0.68 | 0.11 | –3.09 | –7.03, 0.86 | 0.12 |
| Diabetes mellitus (type I and II)‡ | –8.26 | –14.95, –1.57 | 0.02 | –1.93 | –7.66, 3.80 | 0.51 | –0.84 | –6.90, 5.22 | 0.78 |
| Previous episodes of depression‡ | –2.16 | –10.44, 6.11 | 0.61 | –4.95 | –12.03, 2.14 | 0.17 | –7.21 | –14.34, –0.09 | 0.05 |
| Hormonal therapy± | 4.86 | –8.36, 18.08 | 0.47 | 10.46 | –0.66, 21.58 | 0.07 | 3.60 | –8.13, 15.33 | 0.55 |
| Statistical significance p < 0.05 in bold. | |||||||||
| ±, significant difference between the groups. | |||||||||
| *Reference: BT+. ‡Reference: no. EPIC, Expanded Prostate Index Composite questionnaire; BT, brachytherapy. | |||||||||
| Variables | PCS | MCS | ||||
| Regression coefficient B | 95% CI | P | Regression coefficient B | 95% CI | P | |
| Treatment* | –0.31 | –4.18, 3.56 | 0.88 | 1.94 | –1.07, 4.94 | 0.21 |
| Risk group | –1.08 | –4.71, 2.54 | 0.56 | –1.16 | –3.98, 1.65 | 0.42 |
| Months from treatment to survey | –0.31 | –1.15, 0.53 | 0.47 | 0.06 | –0.59, 0.72 | 0.85 |
| Age at time of filling in questionnaire | –0.007 | –0.23, 0.21 | 0.95 | 0.13 | –0.05, 0.30 | 0.15 |
| Heart disease‡ | –3.33 | –6.62, –0.05 | 0.05 | –0.69 | –3.24, 1.87 | 0.56 |
| High blood pressure‡ | –1.47 | –4.03, 1.10 | 0.26 | –0.68 | –2.67, 1.32 | 0.50 |
| Diabetes mellitus (type I and II)‡ | –3.06 | –6.90, 0.77 | 0.12 | –3.55 | –6.53, –0.57 | 0.02 |
| Previous episodes of depression‡ | –2.86 | –7.81, 2.10 | 0.26 | –10.31 | –14.16, –6.46 | < 0.001 |
| Hormonal therapy‡ | –0.37 | –8.02, 7.29 | 0.93 | 3.87 | –2.08, 9.82 | 0.20 |
| Urinary incontinence DSS | 0.06 | –0.02, 0.14 | 0.13 | 0.06 | –0.002, 0.12 | 0.06 |
| Urinary obstruction/irritation DSS | 0.04 | –0.06, 0.14 | 0.39 | 0.11 | 0.03, 0.18 | 0.007 |
| Bowel DSS | 0.15 | 0.06, 0.23 | 0.001 | 0.05 | –0.02, 0.12 | 0.14 |
| Statistical significance p < 0.05 in bold. | ||||||
| *Reference: BT+. ‡Reference: no. PCS, Physical Composite Scores; MCS, Mental Composite Scores; BT, brachytherapy; DSS, Domain Summary Scores. | ||||||
There were no statistically significant associations between the RT modality and the DSSs of the urinary irritative/obstructive or bowel domains (Tables 3 and 4). Increasing level of bowel DSS (i.e. decreasing bowel symptoms) were associated with rising PCS (B = 0.15; 95% CI [0.06 to 0.23], P = 0.001) (Table 4). Increasing DSS levels of the urinary irritative/obstructive domain (decreasing symptoms) were associated with better MCS (B = 0.11; 95% CI [0.03 to 0.18], P = 0.007) (Table 4). Comorbidities of high blood pressure, diabetes mellitus and depression also negatively affected PCS and MCS (Table 4).
Discussion
Comparing BT+ with EBRT, we only observed a statistically and clinically significant difference within the urinary incontinence domain. Men treated with EBRT reported worse symptoms for all single items. The prevalence rates of moderate/big urinary or bowel problems were twice as high after EBRT than after BT+ (P < 0.05). In the regression analysis, increasing levels of bowel DSS (less severe symptoms) were associated with higher PCS (P = 0.001). MCS was significantly improved with reduction of symptoms within the urinary irritation /obstruction domain (P = 0.007). The treatment modality was not associated with PCS or MCS.
Though few EPIC-based studies are published for PCaSs surveyed more than 5-years after BT+, Martinez et al.’s [23] institution-based survey of ≤ 5 years survival contains the most valid data for comparison with our study (Suppl. Table 2). Contrary to our observations, Martinez et al. did not find significant AE differences related to the two treatment modalities. Neither did Parry et al. observe differences between the treatment modalities when they computed clinically important differences between the DSSs established in a population-based survey of UK PCa patients who were surveyed up to 18 months after BT+ or EBRT [24]. Disregarding Freiberger et al.’s [25] figures, which are based on few patients from 2000 to 2003, our BT+ results are relatively similar to recent studies despite the urinary DSSs being higher compared with published results (Suppl. Table 2). However, the results of Freiberger et al. illustrate the increasing advances in BT techniques with time. An explanation for the observed differences may be that BT+ was performed by few highly dedicated radiation oncologists at only one Norwegian institution. During the implantation procedure particular caution was taken to avoid the urethra with strict adherence to dose constraints to the urethral and peri-urethral structures [12].
Our post-EBRT DSS levels were generally lower than those of other studies, especially for the urinary irritative/obstructive domain (Suppl. Table 2). This is particularly apparent when compared with Donovan et al.’s [7] report. However, these patients were initially recruited from a randomized trial. In contrast, EBRT was a ‘routine’ treatment for average patients at Norway’s RT centres. In 2010, RT was applied without the current improvements in RT techniques, hence reduced AEs after EBRT would be anticipated today [26, 27].
Greater severity of post-RT bowel or urinary irritative/obstructive symptoms, but not treatment modality, was associated with decreasing physical and mental QoL, respectively. Surprisingly, urinary incontinence was not significantly associated with QoL. A possible explanation could be that contrary to urinary irritation and bowel dysfunction, slight urinary incontinence alleviated with available hygiene articles, does not limit important components of QoL such as restriction to daily social and leisure activities [28]. Our findings support the importance of avoidance of bowel toxicity by improved RT techniques. Furthermore, PCaSs with major post-treatment urinary irritative/obstructive or intestinal problems should be referred to the specialist health service.
Most EPIC-based surveys assess DSSs. The single items of these DSSs prioritize the average of 3 to 5 functional/dysfunctional aspects, whereas the overall problem with patients’ existing dysfunctions, is covered by only one question. Bearing this in mind, our study’s considerable inter-group differences regarding the prevalence of substantial problems represent important new findings. Substantial bowel and urinary problems were significantly lower after BT+ than the corresponding observations after EBRT. More studies should validate these initial findings and further explore in more detail the associations between dysfunction problems and QoL.
Limitations and strengths
The lack of information about referral routines to BT+ and our exclusion criteria for BT+ represents a considerable selection bias, principally making the comparison between BT+ and EBRT problematic. Pre-treatment assessment of EPIC-based urinary and bowel symptoms or of QoL was not performed in the men in the BT+ group making it impossible to evaluate post-RT changes. Neither had we information of eventual surgical or medical interventions to alleviate bowel or urinary AEs. Finally, conventional RT techniques have been used in our limited-sized EBRT group. On the other hand, the large sample size in the BT+ group, the observation time beyond 5 years’ and the use of the internationally recommended EPIC-26 questionnaire are considered the study’s strength [29].
Conclusion
There is no indication that dose-escalation RT by means of BT increases the rate of long-term adverse effects or decreases QoL. Our exploratory study shows less urinary incontinence and substantial urinary and bowel problems after BT+ compared with EBRT. These observations along with published reports support future randomized trials comparing survival and post-RT adverse effects after BT+ compared with EBRT using current optimal RT techniques.
References
- [1] Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, et al. EAU guidelines on prostate cancer. Eur Urol. 2005;48(4):546–51. doi: 10.1016/j.eururo.2005.06.001
- [2] Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–22. doi: 10.1016/j.radonc.2012.01.007
- [3] Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9–10 prostate cancer. JAMA. 2018;319(9):896–905. doi: 10.1001/jama.2018.0587
- [4] Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–62. doi: 10.1016/j.eururo.2020.09.042
- [5] Aas K, Berge V, Myklebust T, Fosså SD. Comparative survival outcomes of high-risk prostate cancer treated with radical prostatectomy or definitive radiotherapy regimens. Eur Urol Open Sci. 2021;26:55–63. doi: 10.1016/j.euros.2021.01.011
- [6] Wedde TB, Smaastuen MC, Brabrand S, Fosså SD, Kaasa S, Tafjord G, et al. Ten-year survival after high-dose-rate brachytherapy combined with external beam radiation therapy in high-risk prostate cancer: a comparison with the Norwegian SPCG-7 cohort. Radiother Oncol. 2019;132:211–17. doi: 10.1016/j.radonc.2018.10.013
- [7] Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–37. doi: 10.1056/NEJMoa1606221
- [8] Dearnaley D, Griffin CL, Lewis R, Mayles P, Mayles H, Naismith OF, et al. Toxicity and patient-reported outcomes of a phase 2 randomized trial of prostate and pelvic lymph node versus prostate only radiotherapy in advanced localised prostate cancer (PIVOTAL). Int J Radiat Oncol Biol Phys. 2019;103(3):605–17. doi: 10.1016/j.ijrobp.2018.10.003
- [9] Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884–90. doi: 10.1200/JCO.2016.71.7397
- [10] Mohammed N, Kestin L, Ghilezan M, Krauss D, Vicini F, Brabbins D, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):204–12. doi: 10.1016/j.ijrobp.2010.10.009
- [11] D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969
- [12] Lilleby W, Tafjord G, Raabe NK. Implementation of high-dose-rate brachytherapy and androgen deprivation in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83(3):933–9. doi: 10.1016/j.ijrobp.2011.08.028
- [13] Steinsvik EA, Axcrona K, Dahl AA, Eri LM, Stensvold A, Fosså SD. Can sexual bother after radical prostatectomy be predicted preoperatively? Findings from a prospective national study of the relation between sexual function, activity and bother. BJU Int. 2012;109(9):1366–74. doi: 10.1111/j.1464-410X.2011.10598.x
- [14] Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53(1):68–80. doi: 10.1016/j.eururo.2007.09.002
- [15] Fosså SD, Storås AH, Steinsvik EA, Myklebust TA, Eri LM, Loge JH, et al. Psychometric testing of the Norwegian version of the Expanded Prostate Cancer Index Composite 26-item version (EPIC-26). Scand J Urol. 2016;50(4):280–5. doi: 10.3109/21681805.2016.1163617
- [16] Ware J, Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003
- [17] Michigan M. Expanded prostate cancer index composite, Michigan Medicine urology. 2020. Available from: https://medicine.umich.edu/dept/urology/research/epic [cited 1 January 2022].
- [18] Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–8. doi: 10.1016/S0895-4356(98)00109-7
- [19] Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44. doi: 10.1200/JCO.1998.16.1.139
- [20] Skolarus TA, Dunn RL, Sanda MG, Chang P, Greenfield TK, Litwin MS, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015;85(1):101–5. doi: 10.1016/j.urology.2014.08.044
- [21] Stata. Stata 15.1. 2020. Available from: https://www.stata.com/stata15/ [cited 1 October 2021].
- [22] SPSS. IBM SPSS statistics. 2022. Available from: https://www.ibm.com/ibm-spss-statistics-25s [cited 1 October 2022].
- [23] Martínez E, Garin O, Pardo Y, Fernández P, Guix B, Gutiérrez C, et al. Five-year quality of life in patients with high-risk localized prostate cancer treated with external beam radiotherapy alone versus external beam radiotherapy with high-dose-rate brachytherapy boost: a prospective multicenter study. J Contemp Brachyther. 2021;13(1):1–11. doi: 10.5114/jcb.2021.103580
- [24] Parry MG, Nossiter J, Cowling TE, Sujenthiran A, Berry B, Cathcart P, et al. Patient-reported functional outcomes following external beam radiation therapy for prostate cancer with and without a high-dose rate brachytherapy boost: a national population-based study. Radiother Oncol. 2021;155:48–55. doi: 10.1016/j.radonc.2020.10.019
- [25] Freiberger C, Berneking V, Vögeli TA, Kirschner-Hermanns R, Eble MJ, Pinkawa M. Quality of life up to 10 years after external beam radiotherapy and/or brachytherapy for prostate cancer. Brachytherapy. 2018;17(3):517–23. doi: 10.1016/j.brachy.2018.01.008
- [26] Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55(3):241–9. doi: 10.1016/S0167-8140(99)00100-0
- [27] Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, Galvin JM, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932–8. doi: 10.1016/j.ijrobp.2013.07.041
- [28] Fosså SD, Dahl AA. Global quality of life after curative treatment for prostate cancer: what matters? A study among members of the Norwegian Prostate Cancer Patient Association. Clin Genitourin Cancer. 2015;13(6):518–24. doi: 10.1016/j.clgc.2015.07.004
- [29] Sonn GA, Sadetsky N, Presti JC, Litwin MS.Differing perceptions of quality of life in patients with prostate cancer and their doctors. J Urol. 2013;189(1 Suppl.):S59–65; discussion S. doi: 10.1016/j.juro.2012.11.032