SHORT COMMUNICATION
Pseudoporphyria Induced by Voriconazole in a 10-year-old Boy: A Case Report and Review of the Literature
Francesca CAROPPO1,2 , Laura GNESOTTO1,2, Guido MIOSO1,2 and Anna Belloni FORTINA1,2
, Laura GNESOTTO1,2, Guido MIOSO1,2 and Anna Belloni FORTINA1,2
1Pediatric Dermatology Unit, Department of Medicine DIMED, and 2Department of Womens’ and Children’s Health SDB, University of Padova, Padova, Italy. E-mail: francesca.caroppo@outlook.it
Citation: Acta Derm Venereol 2023; 103: adv10286. DOI https://doi.org/10.2340/actadv.v103.10286.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Apr 19, 2023; Published: May 30, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Pseudoporphyria is an unusual photodermatosis similar to porphyria cutanea tarda (PCT). The main clinical features include skin fragility, bullae and vesicle formation, and scarring, on sun-exposed areas (1). Pseudoporphyria may be induced by the frequent use of ultraviolet A (UVA) tanning beds or by chronic renal failure; however, most cases develop secondary to the use of different drugs, including voriconazole (2, 3). We describe here a rare case of pseudoporphyria associated with voriconazole in a paediatric patient.
CASE REPORT
A 10-year-old boy was referred to our Pediatric Dermatology Unit for the appearance of erythema, blisters, and freckling on sun-exposed areas, which began approximately 1 month previously, at the start of the spring season. No recent increase in his daily sun exposure was reported by parents. The patient had a diagnosis of standard-risk pre-B acute lymphoblastic leukaemia in 2018 and had received a hematopoietic stem cell transplant in October 2020, followed by graft-versus-host disease (GVHD). He was currently under treatment with mycophenolate mofetil, prednisone, azithromycin, acyclovir, trimethoprim-sulfamethoxazole and voriconazole. Physical examination revealed erythema with scaling and small brown lentiginous lesions on the dorsal surface of the hands and arms, lateral areas of the neck, face and lips. Mild oedema was observed on his upper extremities (Fig. 1). His parents reported the previous presence of tense bullae containing clear fluid confined to his right helix and dorsal aspects of the hands. On suspicion of photosensitivity reaction related to voriconazole, this drug was immediately discontinued, and no other medication was administered. Additional investigations included a negative autoimmune screening. Serum, urinary and faecal porphyrins were all within normal ranges. Based on clinical and laboratory findings, a diagnosis of pseudoporphyria secondary to voriconazole was made. After 4 weeks, the child achieved complete clinical remission of the skin lesions, with no recurrence in the following weeks. Therefore, the child and his parents were instructed on regular use of sun-protective measures.
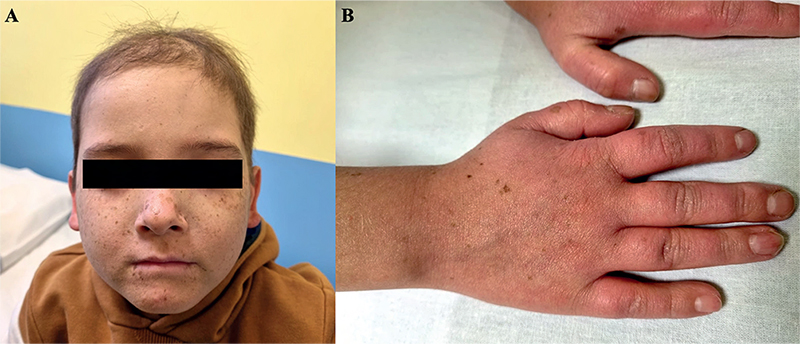
Fig. 1. Skin lesions with erythema, scaling and small brown lentiginous lesions on (A) the face and lips, and (B) the dorsal surface of the hands. Permission is given to publish these photos.
DISCUSSION
Pseudoporphyria is a bullous photodermatosis that imitates the clinical and histological features of PCT (1). Unlike PCT, important features are a lack of hypertrichosis, hyperpigmentation, sclerodermal changes and dystrophic calcification (2). Moreover, pseudoporphyria differs from PCT due to the absence of altered levels of porphyrins in serum, faeces and urine. Histologically, both disorders exhibit subepidermal separation with festooning of the dermal papillae, a mild perivascular infiltration, sporadic periodic acid-Schiff positive thickening of the blood vessel walls and collagen sclerosis (3). Pseudoporphyria has been associated with different medications including non-steroidal anti-inflammatory drugs (NSAIDs), diuretics, tetracycline, oestrogen, retinoids, cyclosporine, flutamide, amiodarone, carisoprodol/aspirin therapy, pyridoxine, and 5-fluorouracil. In addition, there have been reported cases related to haemodialysis, excessive consumption of cola drinks and UVA radiation from tanning beds (3). Voriconazole, another treatment that could be related to pseudoporphyria, is a relatively new triazole antifungal agent that inhibits the fungal enzyme sterol-14- alpha-demethylase, consequently blocking the production of ergosterol. This medication has been used extensively in the management of invasive aspergillosis and antifungal prophylaxis following solid organ transplants or allogeneic blood and marrow transplantation (BMT) (4). Dermatological side-effects related to voriconazole include urticaria, Stevens-Johnson syndrome, toxic epidermal necrolysis, discoid lupus erythematosus, cheilitis, phototoxicity, pseudoporphyria and an increased incidence of squamous cell carcinoma (SCC) in immunocompromised patients (5). The pathogenetic mechanism behind drug-induced pseudoporphyria is not fully clarified. Voriconazole is thought to act similarly to endogenous photoactivated porphyrins or through a protease-mediated damage to vascular endothelium following exposure to sunlight (6, 7). To our knowledge, few cases of pseudoporphyria related to voriconazole in children have been reported in the literature to date, while other photosensitivity reactions were more often observed. In a case series by Goyal et al. (8), the authors reported a group of 40 children who received voriconazole after allo-BMT. Nine (22.5%) patients developed different skin lesions (sunburn-like erythema, pseudoporphyria, linear papulovesicular lesions, severe erosive cheilitis, dermatoheliosis, lentigines) in sun-exposed areas, after the start of therapy (range 1.8–12.5 months). Voriconazole was continued in 4 patients, while it was substituted with fluconazole or posaconazole in the remaining subjects. Topical steroids were prescribed for patients with intense cutaneous reactions. A paediatric case report, by Willis et al. (9), involved a 9-year-old boy with leukaemia, who developed pseudoporphyria and later photo-onycholysis while being treated with voriconazole. However, symptoms improved almost immediately upon drug discontinuation. Frick et al. (10) described 3 cases of voriconazole- induced photosensitivity in immunocompromised children. The drug was switched to oral posaconazole, leading to the complete resolution of skin lesions. In a retrospective case series by Bernhard et al. (11), the authors showed evidence that oral treatment with voriconazole at a dose of ≥ 6 mg/kg/dose twice a day is associated with a high risk of developing phototoxic skin reactions in immunocompromised children. However, dosing recommendations for children from 2 to 11 years of age is 9 mg/kg per dose, not to exceed 350 mg per single dose (12). In cases of suspicion of pseudoporphyria, a careful history including UV light exposure, medications taken, and family history, should be obtained. Moreover, physical examination is crucial to find typical lesions in sun-exposed areas. Routine examinations, including evaluation of blood, urine and faecal porphyrins enable the exclusion of PCT. Other possible different diagnoses comprise erythropoietic protoporphyria, epidermolysis bullosa acquisita, bullous pemphigoid and bullous lupus erythematosus. To complete the diagnostic algorithm, a biopsy specimen and histological evaluation could be useful in case of diagnostic doubts (3). Physicians should be aware of the risk of photosensitivity and photodamage in immunocompromised patients receiving voriconazole, especially in children and adolescents. Accordingly to the literature, identification and removal of the offending agent is the best treatment option for pseudoporphyria, otherwise substitution with other azoles could be considered (1, 8, 10). Skin lesions may improve after several months, and in some cases permanent scarring could develop. Furthermore, education of the patient about the importance of avoiding direct sunlight and artificial tanning beds is crucial; the patient should also apply broad-spectrum sunscreens and use sun-protective clothing for several months after drug cessation.
REFERENCES
- Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed 2007; 23: 29–31.
- Dolan CK, Hall MA, Blazes DL, Norwood CW. Pseudoporphyria as a result of voriconazole use: a case report. Int J Dermatol 2004; 43: 768–771.
- Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol 2001; 44: 100–108.
- Epaulard O, Leccia MT, Blanche S, Chosidow O, Mamzer-Bruneel MF, Ravaud P, et al. Phototoxicity and photocarcinogenesis associated with voriconazole. Med Mal Infect 2011; 41: 639–645.
- Sharp MT, Horn TD. Pseudoporphyria induced by voriconazole. J Am Acad Dermatol 2005; 53: 341–345.
- Keane JT, Pearson RW, Malkinson FD. Nalidixic acid-induced photosensitivity in mice: a model for photoporhyria. J Invest Dermatol 1984; 82: 210–213.
- Dabski C, Beutner EH. Studies of laminin and type IV collagen in blisters of porphyria cutanea tarda and drug-induced pseudoporphyria. J Am Acad Dermatol 1991; 25: 28–32.
- Goyal RK, Gehris RP, Howrie D, Cogley KM, Windreich RM, Venkataramanan R. Phototoxic dermatoses in pediatric BMT patients receiving voriconazole. Pediatr Blood Cancer 2014; 61: 1325–1328.
- Willis ZI, Boyd AS, Di Pentima MC. Phototoxicity, pseudoporphyria, and photo-onycholysis due to voriconazole in a pediatric patient with leukemia and invasive aspergillosis. J Pediatric Infect Dis Soc 2015; 4: 22–24.
- Frick MA, Soler-Palacín P, Martín Nalda A, Guarner ME, Nadal CF. Photosensitivity in immunocompromised patients receiving long-term therapy with oral voriconazole. Pediatr Infect Dis J 2010; 29: 480–481.
- Bernhard S, Kernland Lang K, Ammann RA, Lüer S, Leibundgut K, Diepold M, Aebi C. Voriconazole-induced phototoxicity in children. Pediatr Infect Dis J 2012; 31: 769–771.
- Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob Agents Chemother 2012; 56: 3032–3042.