ORIGINAL REPORT
Use of Pegylated Interferon Alpha-2a in Cutaneous T-cell Lymphoma: A Retrospective Case Collection
Janika GOSMANN1, Rudolf STADLER1, Koen D. QUINT2, Ralf GUTZMER1 and Maarten H. VERMEER2
1University Department for Dermatology, Venereology, Allergology and Phlebology, Skin Cancer Center, Johannes Wesling Medical Center Minden, Ruhr University Bochum, Minden, Germany and 2Department of Dermatology of the Leiden University Medical Center, Leiden, The Netherlands
Mycosis fungoides and Sézary syndrome are rare and largely incurable types of cutaneous T-cell lymphoma with limited therapeutic options. In 1984 Bunn et al. reported that interferon alpha is an efficient monotherapy in cutaneous T-cell lymphoma and 14 years later it was shown in a prospective, randomized trial that a combination of interferon alpha and psoralen plus ultraviolet A therapy (PUVA) is most efficient in the treatment of cutaneous T-cell lymphoma. Since then interferon alpha as single agent or, most often, in combination with phototherapy and/or retinoids has been integrated as standard of care in cutaneous T-cell lymphoma guidelines worldwide. However, production of interferon alpha was discontinued recently worldwide and pegylated interferon alpha-2a (PEG-IFNα) has been used as an alternative therapy. In contrast to numerous interferon alpha studies, only a few studies focusing on PEG-IFNα are available. Therefore, the aim of this study was to conduct a retrospective data collection to report on the efficacy, adverse events and therapy regimens of PEG-IFNα in cutaneous T-cell lymphoma. In 28 patients with cutaneous T-cell lymphoma treated in Germany and in the Netherlands, 36% of patients achieved complete remission, 36% partial remission and 29% stable disease. Eighteen percent of patients developed adverse events during therapy, which led to the discontinuation of PEG-IFNα therapy in 2 patients. The most common concomittant therapies were oral PUVA phototherapy and local radiotherapy. In conclusion, PEG-IFNα, especially in combination with skin-directed therapies, is an effective treatment option for cutaneous T-cell lymphoma in clinical practice.
SIGNIFICANCE
Mycosis fungoides and Sézary syndrome are rare and largely incurable types of skin cancer, that can also affect lymph nodes and blood. Therapeutic options are limited. Recombinant interferon alpha has been used for decades as an effective medication, but was discontinued recently worldwide. Pegylated interferon alpha-2a (PEG-IFNα; Pegasys®, zrpharma Vienna, Austria) has been used as an alternative therapy. This retrospective study analysed the response and adverse events with PEG-IFNα in 28 patients. With 36% of patients achieving complete remission, 36% partial remission, and 29% stable disease, PEG-IFNa presents an innovative treatment option for patients with early as well as advanced CTCL.
Key words: mycosis fungoides; pegylated interferon; Sézary syndrome.
Citation: Acta Derm Venereol 2023; 103: adv10306. DOI: https://doi.org/10.2340/actadv.v103.10306.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 19, 2023; Published: Oct 30, 2023
Corr: Rudolf Stadler, University Department for Dermatology, Venereology, Allergology and Phlebology, Skin Cancer Center, Johannes Wesling Medical Center Minden, Hans-Nolte-Strasse 1, DE-32427 Minden, Germany. E-mail: Rudolf.stadler@ruhr-uni-bochum.de
INTRODUCTION
Mycosis fungoides (MF) and Sézary syndrome (SS) are rare and largely incurable types of cutaneous T-cell lymphoma (CTCL) with limited therapeutic options (1). Interferon alpha (IFNα) is a well-known immunomodulatory modality. Bunn et al. published for the first time, in 1984, that IFNα is an efficient monotherapy in CTCL (2). Current guidelines for the therapy of cutaneous lymphomas, including the European Organisation for Research and Treatment of Cancer (EORTC), the International Society of Cutaneous Lymphomas (ISCL), the European Society for Medical Oncology (ESMO), the British Association of Dermatologists and UK and German guidelines, recommend, among others, the use of IFNα in MF and SS (3–6). The EORTC Cutaneous Lymphoma Task Force (EORTC CLTF) recommends IFNα as first-line therapy alone in MF stage IIB and III and as second-line therapy in MF stage I and IIA (3). The ESMO recommends treatment with IFNα for patients with extensive infiltrated plaques and tumors or patients refractory to skin-directed therapies, in combination with psoralen plus ultraviolet A therapy (PUVA) or other skin-directed therapies (4). IFNα is recommended as first-line treatment in combination with PUVA in MF stage IIB to IVB, or in combination with extracorporal photopheresis or PUVA in Sézary syndrome in the German guideline (6). In prospective and retrospective studies IFNα alone or in combination can be effective in all stages of MF and SS, with overall response rates ranging up to 80% (3, 7). However, published data has the limitation of heterogeneity in treatment schedules, patient selection and methodology and randomized controlled trials are rare (3, 7).
In early 2020 IFNα was withdrawn from the European market and pegylated interferon alpha-2a (PEG-IFNα, Pegasys®) is now increasingly used as an alternative. PEG-IFNα has antiviral, antitumour, immunomodulatory and antiproliferative properties. Compared with non-pegylated IFNα, it has a significantly longer plasma half-life due to its binding to polyethylene glycol, which reduces renal clearance (8, 9). PEG-IFNα is therefore administered subcutaneously only once a week (10). It has been approved for the therapy of chronic hepatitis B and C for more than 20 years (10). Adverse events include anaemia, thrombocytopaenia, hyper- and hypo-thyroidism, anorexia, depression, headache, poor concentration, cough, gastrointestinal complaints, hair loss, dermatitis, arthralgias and myalgias, viral and bacterial infections, fever and fatigue (10).
The aim of this study was therefore to conduct a retrospective data collection on the use of PEG-IFNα in patients with CTCL naive to interferon treatment visiting the dermatology clinics of Leiden University Medical Center (The Netherlands) and Minden University Medical Center (Germany). The study evaluates the therapeutic effect, adverse events, co-medication and treatment regimens of PEG-IFNα.
MATERIALS AND METHODS
A retrospective analysis was performed in the Department of Dermatology at the University Hospitals of Minden and Leiden. All patients treated with PEG-IFNα from January 2020 to October 2022 for CTCL in stage IB to IVAa (MF, SS) were included. The EORTC/ISCL staging system for cutaneous lymphomas was used to evaluate tumour stage (3). For evaluation of therapy response (complete response, partial response, stable disease and progressive disease) the recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC, from Olsen et al. (11), were used. The study was conducted in accordance with the Declaration of Helsinki, and was approved by the ethics committee of Ruhr University of Bochum (approval number 2021-811) and the ethics committee of the University of Leiden (approval number N20.052).
RESULTS
Between January 2020 and October 2022, a total of 28 patients with CTCL, 11 women and 17 men, were treated with PEG-IFNα at the Departments for Dermatology in Minden and Leiden. Details of the study cohort are shown in Table I. Patient age at therapy initiation ranged from 43 to 82 years (range 39 years) and the median age was 65 years (25th percentile = 57, 75th percentile = 73). Most patients (86%; 24/28) had classic MF. Three patients were diagnosed with follicular MF. One patient had Sézary syndrome. At the start of therapy, 6/27 of the patients with MF were in stage IB, 17/27 in stage IIB, 2/27 in stage IIIA, 1/27 in stage IIIB and 1/27 in stage IVA.
The duration of therapy varied from a minimum of 11 weeks to a maximum of 145 weeks (range 134 weeks) and the median duration was 36 (25th percentile = 27, 75th percentile = 65) weeks of therapy. The majority of patients (19/28) were still on therapy at the time of data collection. Reasons for discontinuation were complete remission (56%, 5/9), adverse events (22%, 2/9), inadequate response with stable disease (11%, 1/9) or disease progression (11%, 1/9). In 89% (25/28) of patients, a starting dose of 135 µg once weekly was selected. In 1 obese patient (body mass index (BMI) > 25) a dose of 180 µg was selected, while in 2 very slim patients (BMI < 18.5) a starting dose of 65 µg and 90 µg weekly was selected. In 2 patients, the therapy was reduced from 135 µg to 90 µg once weekly during the course of therapy, due to adverse events.
Monotherapy with PEG-IFNα was used in only 7% (2/28) of patients, whereas, in all other cases, combination therapies were used (26/28). The most frequent combination therapies were PUVA (54%, 15/28) and local radiotherapy (29%, 8/28). A combination with UVB311 nm was given in 7% (2/28). In 25% (7/28) of cases, supplementary local therapy with chlormethine gel (Ledaga®; Recordati, Rare Diseases, Ulm, Germany) was used in addition to interferon therapy. 32% (9/28) patients also received topical steroids (Fig. 1).
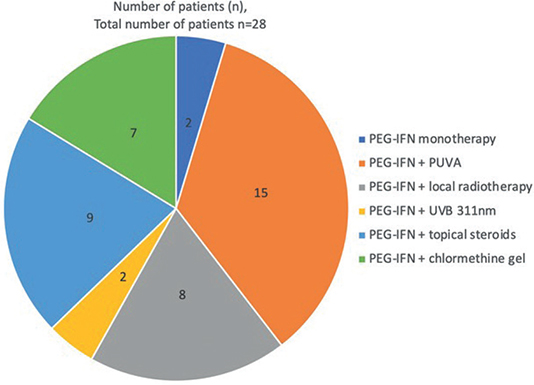
Fig. 1. Therapy combinations in the study cohort (n = 28 patients). PEG-IFN: pegylated interferon alpha-2a; PUVA: psoralen UVA therapy; UVB: ultraviolet B.
Eighteen percent (5/28) of patients developed adverse events during therapy, including gastrointestinal discomfort with diarrhoea, leukopaenia, thrombocytopaenia, lymphopaenia, increase in transaminases, fatigue and weight loss. Adverse events that led to discontinuation of therapy were thrombocytopaenia, lymphopaenia, fatigue and weight loss in 1 patient and gastrointestinal symptoms in another patient. In 2 patients the therapy was reduced from 135 µg to 90 µg 1 × weekly during the course of therapy due to adverse events. Both patients had fewer adverse events after dose reduction, but in 1 of the patients MF plaques increased after dose reduction.
Complete remission was achieved in 36% (10/28), partial remission in 36% (10/28) and stable disease in 29% (8/28) of patients. Patients who received a combination of PUVA and PEG-IFNα achieved complete remission (CR) in 40% (6/15), partial remission (PR) in 53% (8/15) and stable disease (SD) in 7% (1/15). Patients treated with local radiotherapy and PEG-IFNα achieved CR in 38% (3/8), PR in 13% (1/8) and SD in 50% (4/8) (Fig. 2).
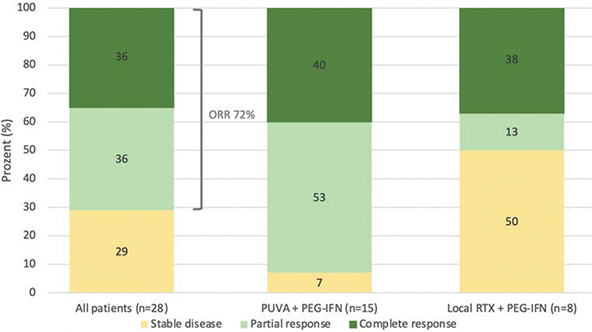
Fig. 2. Therapy response in all patients treated with pegylated interferon alpha-2a (PEG-IFN), in patients treated with a combination of PEG-IFN and psoralen UVA (PUVA), and in patients treated with a combination of PEG-IFN and local radiotherapy. ORR: overall response rate.
Patients with MF stage IB achieved partial or complete response in 50% (3/6) and 50% (3/6) showed stable disease. Patients with classic MF in stage IIB showed partial or complete response in 87% (13/15) and only 13% (2/15) showed stable disease. All patients in stage III (3/3) achieved partial or complete response. All patients with follicular MF (3/3, stage IIB and IVA) achieved stable disease. The only patient with SS achieved a partial response. In the case series, especially patients in stage IIB and III, seem to profit most from the treatment with PEG-IFNα.
Combination therapy of PEG-IFNα with topical steroids, chlormethine-gel, PUVA, UVB 311-nm phototherapy, or local radiotherapy was well tolerated.
DISCUSSION
In this retrospective study 20/28 (71%) of patients showed a partial or complete response to PEG-IFNα combination therapy. In the large majority of cases this therapy could be continued for months or even years without major adverse events. Adverse events were relatively rare, could be managed effectively using dose reduction and led to discontinuation only in a small minority of patients. At the recent presentation at an EORTC/Cutaneous Lymphoma Tumour Group (CLTF) meeting in Madrid in 2022 Mitsunaga et al. reported on the use of PEG-IFNα in 110 patients with CTCL stage IA to IVA2. They found a lower rate of complete remission (12% vs 36%), but a higher rate of partial response (40% vs 36%) than in the current study, possibly due to the lower case numbers in the current study (12). In comparison with Patsatsi et al.’s study PEG-IFNα was only combined with skin-directed therapy in the current study, explaining the high response rates (13). They reported a 75% ORR for stage IB and 55% for all stages, underscoring that PEG-IFNα will belong to standard of care in the treatment of cutaneous T-cell lymphoma.
Prospective trials from Stadler et al. (14) and Chiarion-Seleni et al. (15) evaluated the combination of non-pegylated IFNα and PUVA therapy in patients with MF and found complete response rates from 70% to 74% that were even higher than in the current study, and partial response rates from 6% to 10% that were lower than in the current study. Overall response rate with PEG-IFNα in the current study (72%) was similar to trials with non-pegylated IFN (80%). An international multicentre retrospective follow-up study from the cutaneous Lymphoma International Consortium from 2017 found that IFNα was the second most common first-line treatment in stage IVA1 and also most widely employed as third-line treatment in patients with MF and those with SS (16).
Other haematological malignancies in which PEG-IFNα is used include myeloproliferative deases, such as essential thrombocythemia and polycythemia vera, with overall response rates of 69% and 60% (17). Loss of response to IFN therapy has been reported in patients with hepatitis C, possibly based on the presence of IFN antibodies (18, 19). In the current study no loss of response to IFN in combination with skin-directed therapies was observed.
In a previous study adverse events to IFNα were mild or moderate and affected 55% of patients treated with a combination of IFNα and PUVA (14). Compared with the results of the current study, the number of patients experiencing adverse events (18%) was lower. However adverse events were similar, also mild to moderate, and generally not a reason to stop therapy. As the medication is administered only once a week and can be self-administered by the patient, the effort required on the part of the patient is relatively low.
When interpreting the results of the current study, the shortcomings of a retrospective data collection must be taken into account, which include, above all, a non-standardized and a smaller patient collective, as well as no defined intervals and procedures of assessment of tolerability and efficacy. The influence of co-treatment on therapy response, especially of PUVA used in more than 50% of patients, and of local radiation, must be noted.
This study shown that PEG-IFNα, particularly in combination, with a dose between 135 and 180 µg, is an effective therapy option for treatment of MF and SS, particularly in classic MF stage IIB and III, in the real-world setting and seems not inferior to recombinant IFNα combinations.
ACKNOWLEDGEMENTS
Conflicts of interest: JG received support for travel/meeting participation and honoraria from Recordati. RS: honoraria from 4SC, Abbvie, Galderma, Hoffmann La Roche Innate Pharma, Janssen Pharmaceuticals, Kyowa Kirin International, LEO Pharma, miRagen Therapeutics, Novartis, Recordati, Takeda. RG: honoraria for advice and/or lectures (to person): Almirall, Amgen, Bristol-Myers Squibb, Immunocore, Merck-Serono, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, SUN Pharma, 4SC. Clinical study conduct (to institution): Array Pharma/Pfizer, BMS, Innate Pharma, IO Biotech, Merck-Serono, MSD, Novartis, Philogen, Pierre-Fabre, Roche, Regeneron, Sanofi, Sun Pharma. Project support (to institution): Novartis, Amgen, MerckSerono, SUN Pharma, KyowaKirin, Almirall. Support for travel/meeting participation (to person): Pierre-Fabre, SUN pharma, Boehringer Ingelheim. MHV: Unrestricted grant from: Takeda, Kyowa Kirin International, honoraria from: 4SC, Abbvie, Galderma, Innate Pharma, Kyowa Kirin International, miRagen Therapeutics, Recordati, Taked. KDQ has no conflicts of interst to declare.
REFERENCES
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol 2014; 70: 240–242.
- Bunn PA Jr, Foon KA, Ihde DC, Longo DL, Eddy J, Winkler CF, , et al. Recombinant leukocyte A interferon: an active agent in advanced cutaneous T-cell lymphomas. Ann Intern Med 1984; 101:484–487.
- Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – Update 2017. Eur J Cancer 2017; 77: 57–74.
- Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv30–iv40.
- Gilson D, Whittaker SJ, Child FJ, Scarisbrick JJ, Illidge TM, Parry EJ, et al. British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Br J Dermatol 2019; 180: 496–526.
- Dippel E, Assaf C, Becker JC, von Bergwelt-Baildon M, Bernreiter S, Cozzio A, et al. S2k guideline – Cutaneous lymphomas (ICD10 C82-C86): update 2021. J Dtsch Dermatol Ges 2022; 20: 537–555.
- Spaccarelli N, Rook AH. The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatol Clin 2015; 33: 731–745.
- Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet 2001; 40:539–551.
- Schiller M, Tsianakas A, Sterry W, Dummer R, Hinke A, Nashan D, et al. Dose-escalation study evaluating pegylated interferon alpha-2a in patients with cutaneous T-cell lymphoma. J Eur Acad Dermatol Venereol 2017; 31: 1841–1847.
- EMA Pegasys product information. Last updated November 11, 2022. [accessed 2023 Feb 26]. Available from: https://www.ema.europa.eu/en/documents/product-information/pegasys-epar-product-information_de.pdf.
- Olsen EA, Whittaker S, Willemze R, Pinter-Brown L, Foss F, Geskin L, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood 2022; 140: 419–437.
- Mitsunaga K, Bagot M, Beylot-Barry M, Ram-Wolff C. Real-world study of the use of pegylated interferon alfa for treatment of primary cutaneous T-cell lymphomas: an EORTC CLTF study. Eur J Cancer 2022; 173S1: S1–S58.
- Patsatsi A, Papadavid E, Kyriakou A, Georgiou E, Koletsa T, Avgeros C, et al. The use of pegylated interferon a-2a in a cohort of Greek patients with mycosis fungoides. J Eur Acad Dermatol Venereol 2022; 36: e291–e293.
- Stadler R, Otte HG, Luger T, Henz BM, Kühl P, Zwingers T, et al. Prospective randomized multicenter clinical trial on the use of interferon -2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998; 92: 3578–3581.
- Chiarion-Sileni V, Bononi A, Fornasa CV, Soraru M, Alaibac M, Ferrazzi E, et al. Phase II trial of interferon-alpha-2a plus psolaren with ultraviolet light A in patients with cutaneous T-cell lymphoma. Cancer 2002; 95: 569–575.
- Quaglino P, Maule M, Prince HM, Porcu P, Horwitz S, Duvic M, et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol 2017; 28: 2517–2525.
- Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 2018; 32: 1057–1069.
- Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 2019; 134: 1498–1509.
- Halfon P, Pérusat S, Bourlière M, Bronowicki JP, Trimoulet P, Benhamou Y, et al. ANRS HC16 GAMMATRI Study Group. Neutralizing antibodies to interferon-α and circulating interferon in patients with chronic hepatitis C non-responding to pegylated interferon plus ribavirin re-treated by pegylated interferon-α-2a and ribavirin (ANRS HC16 GAMMATRI substudy). J Med Virol 2010; 82: 2027–2031.