Treatment for hidradenitis suppurativa is diverse, yet frequently unsatisfactory. The aims of this study were to create a reproducible artificial intelligence-based patient-reported outcome platform for evaluation of the clinical characteristics and comorbidities of patients with hidradenitis suppurativa, and to use this to grade treatment effectiveness. A retrospective patient- reported outcome study was conducted, based on online questionnaires completed by English-speaking patients registered to the hidradenitis suppurativa StuffThatWorks® online community. Data collected included patient characteristics, comorbidities and treatment satisfaction. These were recoded into scalable labels using a combination of machine learning algorithm, manual coding and validation. A model of treatment effectiveness was generated. The cohort included 1,050 patients of mean ± standard deviation age 34.3 ± 10.3 years. Greater severity of hidradenitis suppurativa was associated with younger age at onset (p < 0.001) and male sex (p < 0.001). The most frequent comorbidities were depression (30%), anxiety (26.4%), and polycystic ovary syndrome (16.6%). Hurley stage I patients rated topical agents, dietary changes, turmeric, and pain relief measures more effective than tetracyclines. For Hurley stage II, adalimumab was rated most effective. For Hurley stage III, adalimumab, other biologic agents, systemic steroids, and surgical treatment were rated more effective than tetracyclines. Patients with hidradenitis suppurativa often have comorbid psychiatric and endocrine diseases. This model of treatment effectiveness provides a direct comparison of standard and complementary options.
Key words: hidradenitis suppurativa; patient-reported outcome; comorbidities; treatment effectiveness; machine learning; artificial intelligence.
Accepted Mar 21, 2022; Epub ahead of print Mar 21, 2022
Acta Derm Venereol 2022; 102: adv00686
DOI: 10.2340/actadv.v102.1056
Corr: Shany Sherman, Division of Dermatology, Rabin Medical Center – Beilinson Hospital, Petach Tikva 4941492, Israel. E-mail: shanyshnush@gmail.com
SIGNIFICANCE
Patient-reported outcome studies of hidradenitis suppurativa have accurately measured disease severity and quality of life. Treatments are diverse, but frequently unsatisfactory. This worldwide patient-reported outcome study, using a machine learning-based algorithm, provides an overview of hidradenitis suppurativa comorbidities and treatments. The results reinforce current guidelines and support the effectiveness of conservative and complementary measures in earlier-stage disease.
INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease affecting apocrine-gland-rich locations. Patients present with painful nodules that later evolve to deep-seated abscesses. Over time, the recurrent inflammatory process results in fibrosis, scars, sinus tracts, and ulceration. The long mean delay in diagnosis (7.2 years, range 5–14 years) (1‒3) may negatively affect outcome.
The prevalence of HS, reported in the literature, varies from 0.0003% to 1% in registry studies (4, 5) and from 1% to 4% in population-based surveys and clinical observations (6‒9). The comorbid conditions most consistently associated with HS are smoking (70–89%) and obesity (33–51.6%) (2, 10). Other comorbidities include hypertension, dyslipidaemia, polycystic ovary syndrome, thyroid disease, arthropathies, and various psychiatric disorders (11).
According to recent management guidelines for HS, the mainstay of therapy combines lifestyle changes with topical and systemic antibiotic regimens, retinoids, and biologic agents along with surgical procedures (10, 12‒15). Nevertheless, therapy is suboptimal in many cases and often varies according to the clinical experience of the healthcare provider (16, 17).
Patient-focused data provide a comprehensive landscape of patient satisfaction with disease management and the issued treatment. A growing body of evidence supports that patient reports are accurate (18, 19) and, as stated in a recent phase II study, may be even more sensitive than global assessments by physicians (20). Reinforced by a recommendation of the HS ALLIANCE (16) to include patient-reported outcome (PRO) measures in studies, several PRO studies have been conducted for HS to evaluate quality of life, symptoms, pain, disease severity, and effectiveness of specific treatments (21–23). However, none of these studies have evaluated patient satisfaction with a variety of HS treatments, including conservative and alternative treatments, or compared the results with updated guidelines.
The aim of the current study was to build a reproducible artificial intelligence (AI)-based PRO platform for evaluation of the clinical characteristics and comorbidities of patients with HS and for direct comparison, on a single scale, of the effectiveness of the different treatments.
Methods
StuffThatWorks® (STW) (https://www.stuffthatworks.health) is a free-access commercial crowdsourcing platform for patients with chronic medical conditions that aims to provide patients with an easy, intuitive online option to share their personal experience with group members and to self-educate about their condition based on group knowledge. The resulting database of clinical and demographic information allows STW analysts to establish de novo models of treatment effectiveness that are shared with the communities.
This retrospective PRO study was based on data derived from responses to an online questionnaire composed by experienced STW survey designers. The questionnaire was optimized for patients with HS by a certified medical physician (JBL) and a Board-certified dermatologist (SS).
Study population and data acquisition
Data collected by STW are stored unidentified, and a designated number is assigned to each user. As a precondition for joining the STW HS community, patients are asked to provide informed consent for inclusion of their unidentified data in studies. Members of the community are free to disclose only information they want to share and can opt out of the platform at any given time.
The study was approved by the local Institutional Review Board. Members of the STW HS community were reached through online advertising campaigns (Facebook advertisements) and word of mouth. After signing up with a Facebook account, the members completed the 2-part online survey including 44 items. The first part consisted of a generic backbone identical for all STW communities, covering baseline characteristics, diagnosis, symptoms, aggravating factors, treatment experiences, and lifestyle factors. The second part consisted of condition-specific addressing aspects unique to HS (i.e. Hurley stages). The platform was available in English only, and all responders were English speakers. A proprietary STW algorithm was applied to assess the quality and integrity of the reports. In addition, manual sampling was performed. In cases in which the quality of the report was too low (e.g. null answers or nonsense), or the integrity of the report was unreliable, the user was blocked, and the report was excluded from the analyses.
Statistical analysis
Qualitative data derived from open questions, were encoded into a set of nominal categories using a combination of manual and artificial intelligence-based normalization tool created by STW. For example, for the question, “To your knowledge, is there anything that makes your condition worse?”, the answer “milk, processed food, and most probably stress “was coded as “dairy", "processed food", "stress”.
Categorical variables were summarized as median, percentage, and range. χ2 test was performed for comparisons.
Demographic differences among groups based on Hurley stage and variance in treatment adherence, satisfaction with the treating physician, and quality of life (dependent variables) were analysed by Mann–Whitney U test, and χ2 test, as appropriate.
To assess treatment effectiveness, machine learning was used, applying the proprietary STW Bayesian inference model, itself built from 2 models: a model which determines the probability that a certain treatment will be reported as “successful”/”current”/”tried but not successful”, and an improvement model, which determines the weighted efficacy of the treatment for the clinical indicators and overall disease severity. For each user, the reported clinical status before/after treatment was categorized on a scale of 1–5; for “tried but not successful” treatments, improvement was assumed to be 0. The baseline assumptions affecting the results of the improvement model were: (i) a homogeneity-of-improvement approach was used. For example, improvement from a severity score of 5 before treatment to 3 after treatment was considered the same as improvement from a severity score of 3 to 1. (ii) Treatments reported fewer than 10 times were excluded from the analysis. (iii) The more reports of a certain treatment, the more confident the model, whereas treatments with a small number of positive reports were down-weighted by the model. (iv) Current treatment scores were determined according to the key principle that the longer the time on a certain treatment, the higher the weight of the report.
Bonferroni correction was utilized to correct for multiple comparisons. p-values < 0.05 were considered statistically significant.
The analyses were performed with IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. Tableau server s.19: Seattle, Washington; Salesforce.
Results
Characteristics of the study population
The cohort included 1,050 participants (93% female); 974 (93%) were from English-speaking-countries (Fig. S1). The demographic and clinical variables are shown in Table I. The majority of patients (67%) had Hurley II HS. Mean ± standard deviation (SD) age was 34.3 ± 10.3 years at the time of the study and 20.4 ± 8.9 years at onset of HS symptoms. Obesity was reported by 807 patients (77%).

Current age (p < 0.001) and age at onset of symptoms (p < 0.001) differed significantly according to Hurley stage (p < 0.001). Patients with Hurley III HS were older than patients with other stages (p < 0.01, analysis of variance (ANOVA), Scheffe post hoc test), and patients with Hurley I HS had a significantly later age of onset than patients with other stages (p < 0.01). There was no difference in body build or in ethnicity among the 3 Hurley stage groups (Kruskal–Wallis, p = 0.96 and p = 0.48, respectively). Hurley II was more frequent in women (p < 0.001) and Hurley III, in men (p < 0.001).
HS was diagnosed by a physician in 886 cases (83%). There were no differences between patients diagnosed or not diagnosed by a physician in terms of current age, age of presentation, sex, and body build (p > 0.05). However, the distribution of Hurley stages differed (p = 0.001), with twice the rate of Hurley I disease among undiagnosed patients (21.7% vs 10%, p = 0.001). Getting diagnosed was rated as “difficult to extremely difficult” by 614 patients (59%) and “somewhat difficult” by 194 patients (18%); most patients were being followed by a dermatologist (46%) or a general physician (43%) (Fig. S2). General satisfaction with the treating physician(s) also differed among the groups; patients with Hurley III HS reported greater satisfaction than patients with Hurley I (Kruskal–Wallis, p = 0.002). Patients with Hurley III HS were the most adherent to treatment, while patients with Hurley I, the least adherent (Kruskal–Wallis, p = 0.002).
A higher quality of life (on a scale of 1–5) was associated with a lower Hurley stage (Kruskal–Wallis, p < 0.001) (Fig. S3).
Hidradenitis suppurativa and comorbidities
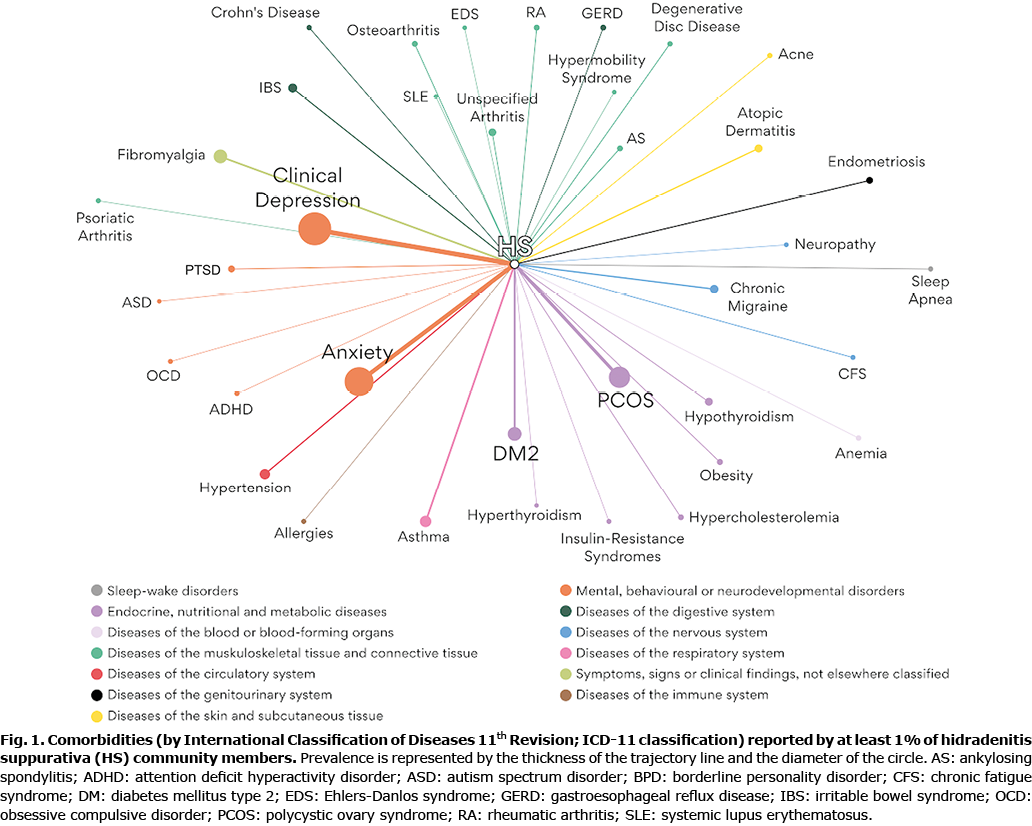
The prevalence rates of comorbidities reported by at least 1% of the cohort are shown in Fig. 1 and Table SI. The most prevalent comorbidities were depression (30%), anxiety (26.5%), polycystic ovary syndrome (16.7%) and type 2 diabetes (10.4%).
Effectiveness of treatments according to Hurley stage
The treatment effectiveness model according to Hurley stages is shown in the boxplots (Fig. 2). For Hurley I HS, turmeric was reported to be the most effective therapeutic agent compared with tetracyclines (p < 0.001), followed by topical treatments (elaborated in Table SII p < 0.001) and dietary changes (p < 0.001). Only 0.2% of patients mentioned both diet and weight loss as tried modalities, suggesting that dietary changes were effective regardless of weight loss. Antibiotics other than tetracyclines (list elaborated in Table SII) had a significant advantage over tetracyclines (p < 0.001). Retinoids, adalimumab and systemic steroids were less effective than tetracyclines (p < 0.001).

For Hurley II, adalimumab was reported to be the most effective therapeutic agent (p < 0.001), followed by rifampin and/or clindamycin (p < 0.001). Other biologic drugs were less effective than tetracyclines (Fig. 2b).
For Hurley III, adalimumab was the most effective agent (p < 0.001), followed by systemic steroids (p < 0.001) and other biologic treatments (p < 0.001). Tetracyclines were reported to be superior to intralesional steroids, retinoids, rifampin and/or clindamycin (Fig. 2c).
The 10 most effective lifestyle modifications, dietary changes and pain relief measures, regardless of Hurley stages, are described in Table SIII.
Discussion
This large-scale international PRO study provides a novel perspective on the characteristics, comorbidities, and treatment-related experience of patients with HS. The results demonstrated that severe disease, expressed by Hurley stage, was associated with older age and male sex, a decline in quality of life, and greater effectiveness of biological treatments. A similar finding of the negative impact of HS severity on quality of life, as assessed by the Dermatology Life Quality Index (DLQI) questionnaire, was reported recently by Krajewski et al. (23). Schrader et al. (24) reported HS severity to be associated with male sex and disease duration. The association between body mass index (BMI) and HS severity is well established (25–27). While, our reported body build finding did not depict such an association, studies show that people tend to underestimate their weight (28, 29).
HS is an underdiagnosed condition, with a mean delay in diagnosis of 7.2 years. Accordingly, the majority (89%) of the current cohort, reported that it was difficult to get diagnosed (1, 2).
Patients diagnosed with Hurley III HS were more satisfied with their primary caregiver and more adherent to treatment. We assume that the management of Hurley III HS necessitates a multidisciplinary team, usually led by a dermatologist, in a tertiary centre with a designated HS clinic, in contrast to Hurley I and II disease, which account for the majority of the HS population.
The current study reinforces previous findings on the prevalence of comorbid conditions in HS, indicating a higher burden of depression and anxiety (~33% of patients) and diabetes ( >10%) (24, 29‒31). Regarding the signals of comorbid conditions, more than 5% of our patients reported fibromyalgia, hypertension, asthma, and irritable bowel syndrome (see Table SI).
This treatment effectiveness model is in line with the suggested Hurley-stage-based management of HS in North American and European guidelines (12, 15, 32). Innovatively, the current model provides validation of the effectiveness of nutritional and pain relief measures using a statistical model. Combination of these with conventional measures in the model reflects their common utilization in real-life HS treatment.
Patients with Hurley I disease reported the greatest benefit from turmeric, dietary changes (e.g. dairy reduction, gluten-free diet (Fig. 2a and Table SIII)) topical treatments (including topical antibiotics and topical steroids) and pain relief measures. Current guidelines recommended topical clindamycin for first-line treatment of mild HS (32), but found insufficient evidence for the benefits of dietary changes (12).
The current model indicated that pain relief agents are effective in mild disease, supporting the Canadian consensus recommendations (17). Validation of effectiveness of pain relief treatments may justify their inclusion as a backbone of HS treatment (33). The advantages of pain relief agents are two-fold, since they also possess an anti-inflammatory effect (34, 35). The North American Clinical Management Guidelines (12) recommend smoking cessation and weight reduction in the management of HS. In the current study, lifestyle changes, including smoking cessation (Table SIII), were superior to oral tetracyclines for Hurley stage I disease (Fig. 1a).
Overall, the range of treatments reported to be effective for Hurley stage I HS (including lifestyle changes, dietary changes, pain relief measures, surgical procedures, and antibiotics) probably reflects the heterogeneity of the inflammatory burden in HS, as suggested by the modified Hurley scoring system (17).
Notably, patients in the current study did not rank intralesional steroid treatment as highly effective, even though it is recommended by the guidelines (32).
Rifampin and/or clindamycin were found to be effective for Hurley stage I and II disease, but were inferior to tetracyclines in Hurley stage III disease. A recent study by van Straalen et al. (36) indicated tetracyclines to be safe and non-inferior in efficacy to rifampin and/or clindamycin regardless of HS severity.
In the current model, adalimumab was most effective for treating Hurley stage II or III HS. Other biological agents, such as ustekinumab, secukinumab, and other tumour necrosis factor (TNF)-alpha inhibitors (infliximab, golimumab, etanercept; Table SII) were perceived to have greater effectiveness than tetracyclines for Hurley III only. Adalimumab is the only Food and Drug Administration (FDA)-approved therapy for HS, and it is the most studied biological treatment for moderate-to-severe HS unresponsive to systemic antibiotics (15). Evidence regarding the effectiveness of other biological treatments is still accumulating (10, 15, 37, 38). Although biological agents are not indicated for Hurley stage I, some patients reported using them, albeit with little effect. We hypothesize that they were prescribed in cases of refractory high-burden inflammatory disease.
Surgical procedures ranked fifth in effectiveness for Hurley I HS and fourth in effectiveness for Hurley III HS, but were not among the 10 most effective treatments for Hurley II HS. These between-group differences might be explained by different patient interpretations of the value of “surgery”. A patient with Hurley stage I could understand “surgery” to mean incision and drainage, whereas to a patient with Hurley stage III, surgery would refer to wide excision of the diseased area leading to cure.
Study limitations
This study has some limitations. The design has an inherent susceptibility to selection bias and recall bias. To minimize the effect of recall bias, we used both open and closed questions. A potential weakness of an online PRO design that should be considered is lack of physician guidance during completion of the online questionnaire. Patients’ understanding or interpretation of the questionnaire items and emotional state while completing the questionnaire might influence their response, thereby affecting the accuracy of the assessment. Most of the study population were English-speaking Caucasian women, probably because of the greater utilization of social media by this population (39) and, consequently, their greater exposure to recruitment advertisements. Therefore, we cannot rule out the possibility of variability in the effectiveness of treatment between males and females. Previous studies have shown that the demographic characteristics and treatment preferences of patients with HS differ by ethnic group and geographical location, e.g. male preponderance in Malaysia, Singapore, and Tunisia (3, 40, 41). Therefore, generalization of the current findings is limited, and further research is required. It is noteworthy that, owing to the low number of reports of weight reduction by responders who found dietary changes to be effective, the interaction between these treatments was not expressly addressed, and further study of the subject is needed.
Conclusion
This large-scale, worldwide PRO study provides a comprehensive picture of the clinical and demographic characteristics of patients with HS and the strong association of the disease with psychiatric and endocrine morbidities. In addition, using this AI-based effectiveness model enabled patient satisfaction with a range of treatments to be compared, and to categorize treatments’ effectiveness according to Hurley stage. The findings of this real-life study may be used as the basis for prospective studies.
ACKNOWLEDGEMENTS
The study was approved by the local Institutional Review Board and was conducted in accordance with the principles of the Declaration of Helsinki.
IRB approval status: 0347-21-RMC.
Conflicts of interest: JBL, RH, and YS are employed by StuffThatWorks® and hold stock options in the company. NK, EH and SS have no conflicts of interest to declare.
References
- Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173: 1546‒1549.
- Bettoli V, Naldi L, Cazzaniga S, Zauli S, Atzori L, Borghi A, et al. Overweight, diabetes and disease duration influence clinical severity in hidradenitis suppurativa-acne inversa: evidence from the national Italian registry. Br J Dermatol 2016; 174: 195‒197.
- Mebazaa A, Ben Hadid R, Cheikh Rouhou R, Trojjet S, El Euch D, Mokni M, et al. Hidradenitis suppurativa: a disease with male predominance in Tunisia. Acta Dermatovenerol Alp Pannonica Adriat 2009; 18: 165‒172.
- Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 2013; 133: 97–103.
- McMillan K. Hidradenitis suppurativa: number of diagnosed patients, demographic characteristics, and treatment patterns in the United States. Am J Epidemiol 2014; 179: 1477‒1483.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008; 59: 596‒601.
- Lachaine J, Miron A, Shear N, Alhusayen R. The prevalence and incidence of hidradenitis suppurativa in Canada: results from a population based survey. Value Health 2016; 19: A123.
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35: 191‒194.
- Jemec GB, Heidenheim M, Nielsen NH. [Prevalence of hidradenitis suppurativa in Denmark]. Ugeskr Laeger 1998; 160: 847‒849 (in Danish).
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009; 60: 539‒561; quiz 562‒563.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020; 82: 1045‒1058.
- Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 2019; 81: 76‒90.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol 2012; 148: 439‒446.
- Slade DE, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg 2003; 56: 451‒461.
- Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29: 619‒644.
- Zouboulis CC, Bechara FG, Dickinson-Blok JL, Gulliver W, Horváth B, Hughes R, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019; 33: 19‒31.
- Alavi A, Lynde C, Alhusayen R, Bourcier M, Delorme I, George R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg 2017; 21: 513–524.
- Rondags A, van Straalen KR, van Hasselt JR, Janse IC, Ardon CB, Vossen ARJV, et al. Correlation of the refined Hurley classification for hidradenitis suppurativa with patient-reported quality of life and objective disease severity assessment. Br J Dermatol 2019; 180: 1214‒1220.
- Rondags A, Volkering RJ, Turcan I, Zuidema YS, Janse IC, Horvath B. The Refined Hurley Patient Questionnaire: an accurate self-assessment instrument for deriving the correct refined hurley stage in hidradenitis suppurativa. Acta Derm Venereol 2019; 99: 703‒704.
- Kimball AB, Sobell JM, Zouboulis CC, Gu Y, Williams DA, Sundaram M, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol 2016; 30: 989‒994.
- Vellaichamy G, Braunberger TL, Jones JL, Peacock A, Nahhas AF, Hamzavi IH. Patient-reported outcomes in hidradenitis suppurativa. G Ital Dermatol Venereol 2019; 154: 137‒147.
- Fernandez JM, Thompson AM, Borgstrom M, Orenstein LAV, Hsiao JL, Shi VY. Pain management modalities for hidradenitis suppurativa: a patient survey. J Dermatolog Treat 2020; 20: 1‒4
- Krajewski PK, Matusiak Ł, von Stebut E, Schultheis M, Kirschner U, Nikolakis G, et al. Quality-of-life impairment among patients with hidradenitis suppurativa: a cross-sectional study of 1795 patients. Life 2021; 11: 34.
- Schrader AM, Deckers IE, van der Zee HH, Boer J, Prens EP. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol 2014; 71: 460‒467.
- Choi F, Lehmer L, Ekelem C, Mesinkovska NA. Dietary and metabolic factors in the pathogenesis of hidradenitis suppurativa: a systematic review. Int J Dermatol 2020; 59: 143‒153.
- Delany E, Gormley G, Hughes R, McCarthy S, Kirthi S, Markham T, et al. A cross-sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 2018; 32: 467‒473.
- Wise J. Many people in UK underestimate their weight. BMJ 2015; 350: h2621.
- Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001–2006. BMC Public Health 2009; 9: 421.
- Reddy S, Strunk A, Garg A. Comparative overall comorbidity burden among patients with hidradenitis suppurativa. JAMA Dermatol 2019; 155: 797‒802.
- Bui TL, Silva-Hirschberg C, Torres J, Armstrong AW. Hidradenitis suppurativa and diabetes mellitus: a systematic review and meta-analysis. J Am Acad Dermatol 2018; 78: 395‒402.
- Machado MO, Stergiopoulos V, Maes M, Kurdyak PA, Lin PY, Wang LJ, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol 2019; 155: 939‒945.
- Hendricks AJ, Hsiao JL, Lowes MA, Shi VY. A comparison of international management guidelines for hidradenitis suppurativa. Dermatology 2021; 237: 81‒96.
- Andersen RK, Jemec GB. Treatments for hidradenitis suppurativa. Clin Dermatol 2017; 35: 218‒224.
- Savage KT, Singh V, Patel ZS, Yannuzzi CA, McKenzie-Brown AM, Lowes MA, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol 2021; 85: 187‒199.
- Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors 2013; 39: 69‒77.
- van Straalen KR, Tzellos T, Guillem P, Benhadou F, Cuenca-Barrales C, Daxhelet M, et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: results of a prospective European cohort study. J Am Acad Dermatol 2021; 85: 369‒378.
- Ghias MH, Johnston AD, Kutner AJ, Micheletti RG, Hosgood HD, Cohen SR. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol 2020; 82: 1094‒1101.
- Takeda K, Kikuchi K, Kanazawa Y, Yamasaki K, Aiba S. Ustekinumab treatment for hidradenitis suppurativa. J Dermatol 2019; 46: 1215‒1218.
- Whitaker C, Stevelink S, Fear N. The use of Facebook in recruiting participants for health research purposes: a systematic review. J Med Internet Res 2017; 19: e290.
- Choi E, Cook AR, Chandran NS. Hidradenitis suppurativa: an Asian perspective from a Singaporean institute. Skin Appendage Disord 2018; 4: 281‒285.
- Loo CH, Tan WC, Tang JJ, Khor YH, Manikam MT, Low DE, et al. The clinical, biochemical, and ultrasonographic characteristics of patients with hidradenitis suppurativa in Northern Peninsular Malaysia: a multicenter study. Int J Dermatol 2018; 57: 1454‒1463.