Currently no treat-to-target framework to guide systemic treatment in adults with moderate-to-severe atopic dermatitis exists. We sought to reach international consensus through an eDelphi process on a core set of recommendations for such an approach. Recommendations were developed by an international Steerng Committee, spanning 3 areas (Guiding Principles, Decision Making, and Outcome Thresholds) and 2 specific time-points; an initial acceptable target at 3 months and an optimal target at 6 months, each based on improvements in patient global assessment plus at least one specific outcome domain. These treat-to-target- orientated recommendations were evaluated by an extended international panel of physicians, nurses and patients. Proposed recommendations were rated using a 9-point Likert scale; for each recommendation, consensus agreement was reached if ≥ 75% of all respondents rated agreement as ≥ 7. Consensus on 16 core recommendations was reached over 2 eDelphi rounds. These provide a framework for shared decision-making on systemic treatment continuation, modification, or discontinuation.
Key words: atopic dermatitis; eDelphi; consensus; surveys and questionnaires; treat-to-target; systemic treatment.
Accepted Jan 21, 2021; Epub ahead of print Jan 21, 2021
Acta Derm Venereol 2021; 101: adv00402.
doi: 10.2340/00015555-3751
Corr: Marjolein de Bruin-Weller, Department of Allergology and Dermatology, UMC Utrecht, National Expertise Center for Eczema, NL-3584 Utrecht, The Netherlands. E-mail: m.s.debruin-weller@umcutrecht.nl
SIGNIFICANCE
Moderate-to-severe atopic dermatitis often requires systemic therapies for disease control. Unlike other conditions, such as psoriasis, at present no treat-to-target framework exists to guide optimal use of systemic therapies in atopic dermatitis. We developed recommendations for such a treat-to-target approach and evaluated these through an eDelphi process with proposals rated by an international panel of physicians, nurses and patients to seek international consensus. After 2 eDelphi rounds, consensus agreement was reached on all recommendations. From this, a clinical algorithm is proposed to guide shared decision-making in a treat-to target approach for systemic treatment in adults with moderate-to-severe atopic dermatitis.
INTRODUCTION
Systemic therapies are an important therapeutic strategy for patients with moderate-to-severe atopic dermatitis (AD) that is not sufficiently controlled by topical therapies. While guidelines list treatment options on the basis of disease severity/activity, they make no recommendation as to treatment order or treatment target, and criteria for the assessment of treatment success are currently not well defined (1, 2). Consequently, decision-making to select a treatment and to guide the assessment of its benefit in individual patients can be complex, and often subjective, and patients may not receive optimal management necessary for disease control.
Treat-to-target is an established strategy in a range of immune-mediated/inflammatory conditions, such as rheumatoid arthritis (RA), spondyloarthopathies, systemic lupus erythematosus, and psoriasis (3–11). In these disease areas, the introduction of biologics led to the development of target-driven treatment algorithms in which specific target goals for treatment response are used to guide decisions to continue, discontinue, or modify treatment(s). This approach provides a framework to support treatment choices made using shared clinical decision-making with patients, including discussion of relevant risks/benefits of existing or alternative treatments.
The use of a similar treat-to-target approach for systemic treatment in AD would seem to be a promising strategy to optimize patient outcomes (12). However, at present, no such framework exists. The objective of this evidence-based expert consensus process was to develop a core set of recommendations for treatment targets, measures and timing, in order to guide the use of systemic therapies in adults with AD in accordance with recommended guidelines (1, 2).
MATERIALS AND METHODS
To establish treatment targets for AD, a consensus-building study was conducted among dermatologists, dermatology nurses and patient representatives through an eDelphi method. Key aspects of this process are described below (a more detailed description is available in Appendix S1).
In the pre-Delphi phase, the available evidence relevant to disease assessment, patient characteristics, and treatment pathways in moderate-to-severe AD in adults was reviewed, discussed and summarized in a series of meetings. A Steering Committee was then established, comprising experts from across Europe (MdB-W, TB, MD, JH, GG, AP, M-A R, J-F S, SW), Canada (RB, C-HH), Australia (PF, SS) and Japan (NK); members were selected on the basis of experience and expertise in treating AD, publication record, participation in clinical trials on AD, and/or participation in comparable consensus activities, such as HOME (13), and TREAT (14), or AD guideline development. All members of the Steering Committee declared potential conflicts of interest.
The Steering Committee then convened over a series of meetings to establish a core set of candidate statements that could inform a treat-to-target approach; these were then included in a survey questionnaire to be examined in an eDelphi process. This was facilitated by an independent methodology expert appointed to guide the eDelphi process and establish consensus rating and agreement criteria. Eligibilty for inclusion in an international extended panel for the eDelphi process was also established. An overview of the approach is shown in Appendix S1; Fig. S1.
eDelphi survey questionnaire development
A list of statements was drafted by the Steering Committee, falling within 3 broad areas pertinent to a treat-to-target approach: Guiding Principles, Decision Making, and Outcome Thresholds. For outcome assessment, a multidimensional approach was adopted, including a range physician-reported and patient-reported outcome measures to provide flexibility and ensure clinical utility; Patient self-reported Global Assessment of disease severity (PtGA), Eczema Area and Severity Index (EASI); SCORing Atopic Dermatitis (SCORAD); Dermatology Life Quality Index (DLQI); Patient-Oriented Eczema Measure (POEM); and Peak Pruritus Numerical Rating Scale (NRS). This broad range of validated instruments reflects the fact that no single outcome assessment tool can capture the entire benefit of a treatment (12), and that physician assessments of clinical signs and patient-oriented assessments are both important (12, 15).
For each instrument, a threshold was proposed for 2 assessment time-points; the initial threshold to be attained at 3 months, and the second to be attained at 6 months, chosen on the basis that they represent an appropriate treatment period for assessing therapeutic response, and align with typical consultation schedule patterns.
eDelphi participants and recruitment
Panel participants were selected to provide representation of key stakeholder groups; physicians and specialist nurses with experience in the management of patients with moderate-to-severe AD, and patients/patient association representatives. In keeping with other similar initiatives, panellists were recruited from a wide range of countries and geographical locations (3–5, 16, 17). Further details on participant selection are available in Appendix S1.
Eligible candidate panel participants were invited directly by Steering Committee by email, explaining the project’s aim and methodology and requesting their agreement to participate. Final selection was influenced by the need to achieve a balanced and pragmatic geographical spread. In addition, in part due to recruitment limitations in selecting appropriate nurses and patient representatives, but also with the aim of generating a core dataset that would have the greatest value and acceptance by and for dermatologists, it was agreed that the majority of panellists would be physicians. Following agreement to participate, to avoid any potential influence on subsequent participant responses in the eDelphi, no other communication or educational activities with panel participants occurred prior to formal participation in the eDelphi process.
The final extended panel comprised 87 participants; the 14 members of the Steering Committee (all physicians), 60 additional physicians, 3 nurses, and 10 patient representatives. The panel included members from 28 different countries, representing most of mainland Europe, Australia, Japan, and Canada (Appendix S1; Table SI).
eDelphi process and definition of consensus
The eDelphi questionnaire consisted of core statements accompanied by supporting information; all panel participants received identical questionnaires. Participants were asked to rate each of the statements using a 9-point Likert scale ranging from 1 = “strongly disagree” to 9 = ”strongly agree”. Consensus on any given statement required 75% or more of all participants to rate their level of agreement as 7, 8, or 9 (a “consensus in” approach). Applying this rule across all participants, rather than to each individual stakeholder group, reduced the risk that a lower level of agreement in the smaller stakeholder group could exert undue influence on the overall result.
For round 1 of the eDelphi, the statements were rated, and results and feedback gathered for analysis. Those statements that met the criteria were considered to be agreed and were not available for voting in subsequent eDelphi rounds. Those statements that failed to reach agreement were reviewed and revised by Steering Committee members, after considering the voting scores and comments, and then submitted for a new eDelphi voting round (Appendix S1; Table SII). In each subsequent voting round, participants were able to view the voting results and anonymized comments for the previous eDelphi round.
Questionnaire distribution, data entry and collection of respondent ratings and feedback, was performed on a dedicated, password-protected, online platform (www.t2tconsensus.com), independently managed by a medical communications agency (IntraMed, Milan, Italy). All participant responses were anonymized (using unique respondent identification numbers), although their stakeholder category was recorded; participant anonymity was maintained throughout the eDelphi process and subsequent discussions. Descriptive data analysis was performed using Microsoft Excel.
In line with similar externally supported consensus projects, the project sponsor was not present during the Steering Committee discussions on statement development, and had no involvement in the conduct of the eDelphi and subsequent consensus process.
RESULTS
eDelphi rounds
A total of 16 statements (and a total of 22 different statement items) were presented and achieved agreement over 2 eDelphi rounds. The percentages of respondents who indicated agreement (rating 7 or higher on the 9-point Likert scale) for the final agreed statements are presented in Table I. For eDelphi round 1 (conducted on 24 June 2019), 57 panellists participated (representing 65.5% of all available panellists), including: 44 physicians (59.5% of panel physicians): 3 nurses (100%); and 10 patients/patient association representatives (100%). Consensus agreement was reached for a total of 11 statements; items 1–5, 7, 9, 10, 11b, 13b and 15b; with aggregated agreement from the overall panel ranging from 77.2 to 98.2% for these statements achieving consensus after round 1 (Table I).

For those statements for which agreement was not achieved (with aggregated agreement ranging from 57.8% to 71.9%), revision and subsequent re-voting was performed. Revisions were related mostly to language framing the proposed statements, and the target threshold values remained unchanged. Statement revisions and additional explanatory notes available for eDelphi round 2 are shown in Appendix S1; Table SII.
In round 2 (conducted on 7 August 2019), a total of 52 participants responded (59.8% of all available panellists); 43 physicians (58.1%), 2 nurses (66.7%), and 7 patients/patient association representatives (70%). All remaining statements and items examined in round 2 after revision (6, 8, 11a, 12a, 12b, 13a, 14a, 14b, 15a, 16a, and 16b) met the ≥75% consensus agreement criteria in this second eDelphi round; with aggregated agreement from the overall panel ranging from 75.0% to 90.4% for these statements (Table I). Some variation in agreement across the different panel categories was observed; while all recommendations achieved ≥ 75% consensus by dermatology and nursing panel participants, a number of statements failed to achieve agreement by patient/patient association representatives.
Consensus meeting and round-table discussion
A face-to-face meeting of the Steering Committee was held (in Frankfurt on 25 October 2019), in which the eDelphi survey results were presented. The agreed recommendations consider decision-making at 3 and 6 months, where an initial acceptable target and subsequent optimal treatment target can be used within the context of a clinical algorithm to guide use of systemic therapies in a treat-to target approach for moderate-to-severe AD (Fig. 1). This algorithm was discussed and endorsed by the Steering Committee.
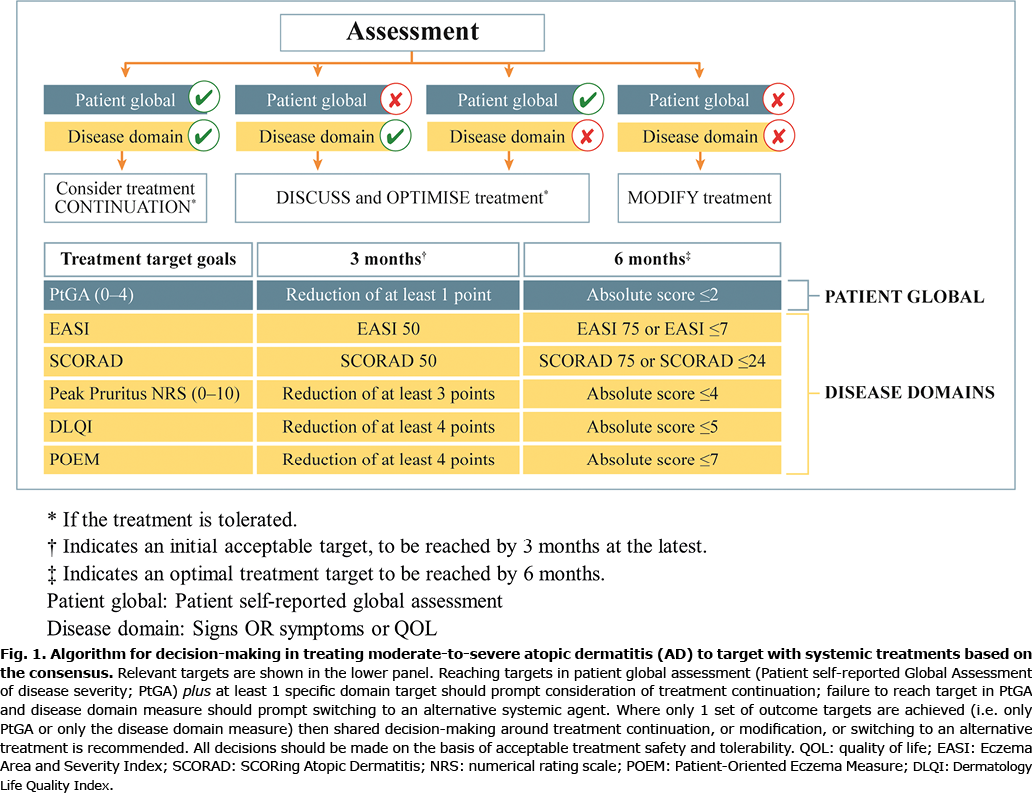
In this algorithm, decisions for continuing or changing therapy are based on changes in the patient’s global assessment plus changes in at least one specific domain measured with the help of validated outcome assessment instruments (PtGA, EASI, SCORAD, Peak pruritis NRS, DLQI and POEM). Reaching targets in both patient global response plus at least one specific domain target should prompt consideration of treatment continuation. Failure to reach targets in both patient global assessment and disease domain measures should prompt transition to an alternative treatment. Where only one set of outcome targets is achieved, shared decision-making will guide continuation, modification, or a change of treatment. In addition, as treatment safety and tolerability are cardinal considerations, these should be taken into account at each decision point.
DISCUSSION
Through an eDelphi consensus process involving an international group of practising physicians with long-standing experience in treating patients with moderate-to-severe AD, along with nurses and patient representatives, agreement was reached on a core set of statements related to treat-to-target outcomes in patients with moderate-to-severe AD requiring systemic therapy. Note that our approach assumes that adjunctive use of topical therapies (including topical corticosteroids or topical calcineurin inhibitors) may continue, or indeed evolve. While this may have some impact upon disease response, the 3 principal clinical decisions around systemic therapy (treatment continuation; switching to an alternative systemic agent; or shared decision-making on treatment continuation or modification or switching) would still apply.
While decisions on treatment strategies can be made at every consultation, 2 specific decision-points were proposed, at 3 and 6 months, representing time-points where an initial acceptable target, and then an optimal treatment target, should be met. If the targets are not met at these time-points, then treatment should be re-evaluated, and optimization or alternatives considered. These 3- and 6-month time-frames correspond well with typical routine visit schedules and may represent a practical treatment period for assessing therapeutic response. However, these are suggested, and some flexibility is anticipated depending on physician preference and the specific treatments being used.
A range of outcome assessments and instruments were chosen in order to provide flexibility and utility across a range of clinical settings and diverse geographical locations, and to balance observer-only and patient-experienced measures. Despite a lack of a standardized definition on the concept and the wording/phrasing, a satisfactory change in the patient global response (e.g. PtGA) was considered to be important, as it represents the patient’s broad perception of disease activity, and is simple and feasible. This is in line with results from a recent research prioritization exercise conducted by the new HOME clinical practice initiative that identified a patient global assessment (PtGA) instrument as being of high importance to investigators, clinicians, and patients in the clinical practice setting (18). However, there was consensus that at the same time, at least 1 specific key domain measured objectively with validated and recommended instruments (18), e.g. SCORAD, EASI, DLQI, Peak Pruritus NRS, or POEM, should show a satisfactory improvement, with the flexibility to use 1 or more, depending on the physician’s and the patient’s preference.
The threshold targets guiding treatment decisions (Fig. 1) were closely considered during survey development, throughout the eDelphi process, and in subsequent round-table discussions. The targets are based on what was considered to be clinically relevant absolute threshold values or percentage reductions. Many targets recommended for initial assessment at 3 months are reductions that represent acceptable improvements, whereas the later 6-month thresholds were set as the optimal target threshold values. Similar to our earlier comments on time-points, the outcome tools and target thresholds are suggested; in reality some flexibility may be anticipated. Choice of outcome instrument may, for example, be influenced by physician and patient preference as well as the specific agent used. In principle, the choice of outcome instruments and specific domain to be used for clinical decision-making would be the decision of the treating physician and the patient (on the basis of agreed individual treatment goals). In addition, certain outcome instruments may be preferred, or even necessary when specific agents are being used, either for reimbursement purposes (where for example, it may be necessary to document EASI responses) or where local protocols apply. However, from a response target threshold perspective, we believe that the thresholds we propose could and perhaps should apply to any systemic AD treatment, regardless of the specific systemic agent. While the time-points and treat-to-target thresholds we propose are intended to help guide treatment decisions in the real-life setting, these time-points and targets are consistent with those used in clinical studies.
The recommended treatment target goals have some similarities to those treat-to-target goals developed via consensus for psoriasis, but some differences exist, in part due to different outcome instruments (8–11, 19, 20). Both the long-established European consensus and later Australian consensus defined psoriasis treatment goals on the basis of changes in the Psoriasis Area and Severity Index (PASI) and the DLQI score (8–10). In contrast, the Canadian consensus uses a single outcome instrument, the Physician Global Assessment (PGA) (11). In the USA, the National Psoriasis Foundation also uses a single outcome tool, evaluating body surface area (BSA) changes from baseline at 3 and/or 6 months (19). Most recently, a Belgian consensus publication used a multidimensional approach, where ideal and acceptable treatment targets after 12 weeks were determined using a range of instruments including PASI, PGA and DLQI (20). While, to some extent, our approach and consensus treatment goals in AD align better with this latter multidimensional approach, in general they are broadly consistent with the principles seen in each of these treat-to-target outcomes in psoriasis. Our inclusion of a symptom specific measurement in our AD treat-to-target framework, the Peak Pruritus NRS, reflects the capacity to monitor itch as an important treatment goal from the patients’ perspective.
Most treat-to-target initiatives in dermatology generate consensus principally from physicians only. In contrast, the present project also included active involvement of nurses and patient representatives, one of the few to do so, although the recent Belgian treat-to-target consensus in psoriasis also included patient input (20). Although the majority of panel participants were physicians, we believe that including representation from patients and nurses has value in developing treat-to-target aims in AD, even if the numbers were relatively low.
Our consensus initiative has some limitations. Although panel participants were drawn from a wide range of countries, the great majority were from Europe, with smaller numbers from Australia, Canada and Japan. Furthermore, the response rate for the first eDelphi round was 65% of all possible participants, with some subsequent drop-out in round 2 (60% response rate). Anonymity was maintained throughout the eDelphi process, which can allow more freedom in participant responses. However, such anonymity does not allow confirmation of specific panelist participation, nor changes in specific participant’s voting. Our extended panel recruitment included 3 different stakeholder groups (physicians, nurses and patient groups). While all recommendations achieved consensus in physicians and nursing stakeholder groups, a lower level of agreement was evident for some statements from patient/patient representatives. This may be due to lack of familiarity with the assessment instruments that were proposed, and to the relatively low number of such participants recruited, but it may be that genuine differences in opinion exist. We also accept that the relatively low number of nurse and patient/patient representative participants (in particular that for nurses) is a study limitation.
Another limitation is the absence of long-term target outcomes in the recommendations and treatment algorithm. Clearly these outcomes are important, and their inclusion would be desirable. However, at the time of our initiative, specific validated instruments were not yet available for use in everyday practice. Some instruments have subsequently been validated, such as the Atopic Dermatitis Control Test (ADCT) (21, 22), and Recap of Atopic Eczema (RECAP) (23). Their inclusion could be considered in future updates of this document. Finally, some clinically relevant aspects have not been addressed in the present process. One such example, beyond the scope of the current consensus process, could be how to account for the potential impact of existing or additional adjunctive topical therapies on target outcomes, and how this may influence systemic treatment decisions.
As with many such treat-to-target proposals, the evidence-base for specific recommendations is at present sparse. The aim of this study has been to provide a framework based on expert opinion and informed by extensive clinical experience, and to seek agreement across an extended panel via an eDelphi process. We believe that this represents a starting point and foundation, which can inform and stimulate debate across the wider dermatology community. Use of these recommendations and algorithm may also be a helpful aid to monitoring clinical practice and could be a valuable tool for clinical audit.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the key role of Christian Apfelbacher (Institute of Epidemiology and Preventive Medicine, University of Regensburg, Regensburg, Germany) for his input in developing and facilitating the eDelphi process. We wish to thank Valentina Guasconi and Stephen McGrath (both from IntraMed Communications, Milan, Italy) for their support in managing the eDelphi process and facilitating the preliminary advisory boards, subsequent consensus meetings and allied logistics. Iain O’Neill (independent medical writer) provided support in manuscript development (funded by Sanofi Genzyme). We also wish to thank all the healthcare professionals and patient advocates who participated in the eDelphi consultation groups and agreed to be formally acknowledged (listed in Appendix S1; Table SIII).
Funding sources. The logistics of the consensus meetings and eDelphi process were supported by Sanofi Genzyme. Sanofi Genzyme had no influence on the project methodology or interpretation of results or on the content and viewpoints expressed in this manuscript. Manuscript development was commissioned by TREATGermany with the support of an unrestricted grant from Sanofi Genzyme.
Disclosure of potential conflicts of interest. MdB-W is co-principal investigator of the Dutch BioDay Registry and serves as a principal investigator in a number of multi-centre clinical trials in atopic dermatitis sponsored by a wide range of pharmaceutical companies; has attended advisory boards and educational events sponsored by Sanofi-Genzyme, Regeneron, AbbVie and Galderma; has received institutional research grants from Sanofi Genzyme; and has performed consultancy roles with Sanofi-Genzyme, Regeneron, LEO Pharma, Eli Lilly, Abbvie, Pfizer, Galderma and UCB, outside the submitted work. TB has received speaker and consultancy fees, and has received institutional research grants from Alk-Abelló, Celgene, Mylan, Novartis, and Phadia-Thermo, outside the submitted work. RB is an employee and shareholder of Innovaderm Research; has received speaker and consultancy fees, and has received institutional research grants from AbbVie, AntibioTx, Aquinox Pharma, Arcutis, Arena Pharma, Asana BioSciences, Bellus Health, Boehringer-Ingelheim, Brickell Biotech, Dermavant, Celgene, Dignity Sciences, Eli Lilly, EMD Serono, Galderma, Glenmark, GSK-Stiefel, Incyte, Kiniksa, Kyowa Kirin, Leo Pharma, Neokera, Novan, Pfizer, Ralexar, Regeneron Pharmaceuticals, Sanofi Genzyme, Sienna and Vitae, outside the submitted work. MD has received speaker fees and served as a consultant, and has received institutional research grants from AbbVie, Almirall, Eli Lilly, Galapagos, LEO Pharma, Meda Pharma, Pfizer, Pierre Fabre, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme, outside the submitted work. PF has attended advisory boards for Abbvie, Amgen, Celgene, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, Galderma, GSK, Mayne Pharma, and Sanofi; has served as a consultant to Janssen, Leo Pharma, Lilly, Novartis, Pfizer, UCB Pharma, BMS, Galderma, Hexima, Mayne Pharma, and Roche; has acted as an investigator in clinical trials for Abbvie, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, Astra Zeneca, BMS, Botanix, Celtaxsys, CSL, Cutanea, Dermira, Galderma, Genentech, GSK, Hexima, Regeneron Pharmaceuticals Inc., Roche, and Sanofi; has received institutional research grants from Abbvie, Amgen, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, and Sanofi; has received speaker fees or other honoraria from Abbvie, Celgene, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, UCB Pharma, Valeant, Galderma, GSK, Roche, and Sanofi; and has received travel expenses for attendance at educational meetings from Abbvie, Janssen, Leo Pharma, Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, Roche, and Sanofi, outside the submitted work. GG has been principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Bristol-Meyers Squibb, Celgene, Celltrion, Eli-Lilly, Genzyme, Leo Pharma, Menlo therapeutics, Novartis, Pfizer, Regeneron, Samsung, Sandoz and Sanofi, outside the submitted work. JH has attended advisory boards for Sanofi Genzyme and Elli Lilly; has acted as an investigator in clinical studies sponsored by Novartis, Amgen, Pfizer, Elli Lilly, and Fresenius Kabi; has served as a consultant to Leo Pharma and Fresenius Kabi; and has received speaker fees from Janssen, Eli Lilly, AbbVie, Novartis, UCB, Sanofi, Aventis, L´Oreal, Sandoz, and Celgene, outside the submitted work. C-HH has received honoraria as a speaker/consultant for Abbvie, Amgen, Bausch Health, Celgene, Eli Lilly, Galderma, Glaxo-Smith-Kline, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi Genzyme, and UCB; and has received grants as an investigator from Abbvie, Amgen, Bausch Health, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Dermavant, Eli Lilly, Galderma, Glaxo-Smith-Kline, Incyte, Janssen, Leo Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, and UCB, outside the submitted work. NK has received honoraria as a speaker/consultant for Sanofi, Maruho, Avvi, Ely-Lilly Japan, Taiho Pharmaceutical, Jansen Pharma, Mitsubishi Tanabe Pharma, and has received grants as an investigator from Sanofi, Ely-Lilly Japan, and Leo Pharma, outside of submitted work. AEP has acted as an advisor and speaker for Sanofi, Abbvie, Lilly, Novartis, Almirall, La-Roche Posay, Leo, UCB, Novartis, and Janssen, outside of submitted work. M-AR has served as the principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Bristol-Meyers Squibb, Celgene, Eli-Lilly, Leo Pharma, Novartis, and Sanofi, outside of submitted work. SS has received has received honoraria as a speaker/consultant for Sanofi, and has received institutional reseach grants as an investigator from Sanofi, outside of submitted work. JFS has served as a consultant and received speaking fees at educational events for Sanofi-Genzyme, Regeneron, Abbvie and Novartis; and has served as the principal investigator in clinical trials sponsored by AbbVie, Amgen, Eli-Lilly, Leo Pharma, Novartis and Pfizer, outside of submitted work. SW is the co-principal investigator of the German Atopic Eczema Registry (TREATGermany) and coordinator of the EU/IMI project BIOMAP (BIOMarkers in Atopic dermatitis and Psoriasis) projects and is an investigator in a number of clinical trials in atopic dermatitis and psoriasis sponsored by a wide range of pharmaceutical companies; has received institutional research grants from Sanofi Genzyme, LEO Pharma and L’Oreal; has acted as a consultant for Sanofi-Genzyme, Regeneron, LEO Pharma, Eli Lilly, Abbvie, Pfizer, Incyte and Novartis; and has lectured at educational events sponsored by Sanofi-Genzyme, Regeneron, LEO Pharma, AbbVie and Galderma, outside of submitted work.
REFERENCES
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682.
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850–878.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010; 69: 631–637.
- Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15.
- Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis 2018; 77: 819–828.
- Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018; 77: 3–17.
- van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrom K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014; 73: 958–967.
- Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, Nast A, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011; 303: 1–10.
- Mrowietz U. Implementing treatment goals for successful long-term management of psoriasis. J Eur Acad Dermatol Venereol 2012; 26: 12–20.
- Baker C, Mack A, Cooper A, Fischer G, Shumack S, Sidhu S, et al. Treatment goals for moderate to severe psoriasis: an Australian consensus. Australas J Dermatol 2013; 54: 148–154.
- Gulliver W, Lynde C, Dutz JP, Vender RB, Yeung J, Bourcier M, et al. Think beyond the Skin: 2014 Canadian Expert Opinion Paper on Treating to Target in Plaque Psoriasis. J Cutan Med Surg 2015; 19: 22–27.
- Thyssen JP, Vestergaard C, Deleuran M, de Bruin-Weller MS, Bieber T, Taieb A, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: e839–e842.
- Leshem YA, Chalmers JR, Apfelbacher C, Furue M, Gerbens LAA, Prinsen CAC, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the Harmonising Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol 2020; 82: 1181–1186.
- Vermeulen FM, Gerbens LAA, Bosma AL, Apfelbacher CJ, Irvine AD, Arents BWM, et al. TREatment of ATopic eczema (TREAT) Registry Taskforce: consensus on how and when to measure the core dataset for atopic eczema treatment research registries. Br J Dermatol 2019; 181: 492–504.
- Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a Consensus document. Section II: Tools for Assessing the Severity of Atopic Dermatitis. J Cutan Med Surg 2018; 22: 10S–16S.
- Gerbens LA, Boyce AE, Wall D, Barbarot S, de Booij RJ, Deleuran M, et al. TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries. Trials 2017; 18: 87.
- Gerbens LAA, Apfelbacher CJ, Irvine AD, Barbarot S, de Booij RJ, Boyce AE, et al. TREatment of ATopic eczema (TREAT) Registry Taskforce: an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema photo- and systemic therapy registries. Br J Dermatol 2019; 180: 790–801.
- Chalmers JR, Thomas KS, Apfelbacher C, Williams HC, Prinsen CA, Spuls PI, et al. Report from the fifth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2018; 178: e332–e341.
- Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol 2017; 76: 290–298.
- Grine L, de la Brassinne M, Ghislain PD, Hillary T, Lambert J, Segaert S, et al. A Belgian consensus on the definition of a treat-to-target outcome set in psoriasis management. J Eur Acad Dermatol Venereol 2020; 34: 676–684.
- Simpson E, Eckert L, Gadkari A, Mallya UG, Yang M, Nelson L, et al. Validation of the Atopic Dermatitis Control Tool (ADCT(c)) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol 2019; 19: 15.
- Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin 2020; 36: 367–376.
- Howells L, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol 2020; 183: 524–536.