Chlormethine is a bifunctional cytotoxic alkylating agent that binds to DNA, resulting in cell death (apoptosis). Chlormethine (also known as mechlorethamine) gel (CL gel) was approved in the European Union in 2017 and was first used in 2019. The aim of the study is to examine evidence regarding the efficacy and safety of chlormethine gel in everyday clinical experience from a cutaneous lymphoma centre. Twenty-three patients with stage IA–IIB mycosis fungoides received chlormethine gel between September 2020 and May 2021. All patients started by applying the gel daily and were monitored every month. At 1, 3, 6 and 9 months, 0%, 43.47%, 56.52% and 65.22% of patients, respectively, achieved an overall response. Five out of 23 patients (21.73%) achieved near complete response at a mean time of 6 months. Chlormethine gel was given as monotherapy in 12 patients (52.17%), and in addition to systemic treatments (methotrexate and peginterferon alpha-2a) in 11 patients (47.82%). Adverse events (AE) were recorded in 43.47% of patients, but only 3 discontinued treatment, due to dermatitis. Scale down of the treatment to application 3-times per week led to better patient compliance. This study shows that chlormethine gel is effective and safe in patients with mycosis fungoides with different types of skin lesions.
Key words: chlormethine; cutaneous lymphoma; mycosis fungoides; modified Severity Weighted Assessment score; mSWAT.
Accepted Feb 23, 2022; Epub ahead of print Feb 23, 2022
Acta Derm Venereol 2022; 102: adv00730.
DOI: 10.2340/actadv.v102.1095
Corr: Evangelia Papadavid, National and Kapodistrian University of Athens, Medical School, Attikon General University Hospital, 1 Rimini Street, Chaidari, GR-12462 Athens, Greece. E-mail: papadavev@yahoo.gr
SIGNIFICANCE
Only a limited number of studies evaluating efficacy and side effect profile of chlormethine gel in patients with mycosis fungoides, are available. Twenty-three patients were treated with chlormethine gel, either as monotherapy or in combination with other systemic agents. In our real-life data, chlormethine is a well-tolerated and safe treatment modality for all MF lesions including patches, plaques and tumors, and we have observed better and faster response rates in patients with early and limited disease compared to extensive and tumor stage patients.
INTRODUCTION
Primary cutaneous lymphomas (PCLs) are defined as non-Hodgkin lymphomas that present in the skin with no evidence of extracutaneous disease at the time of diagnosis. Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL), accounting for 65% of CTCLs. The mean age of diagnosis of MF is in the range 55–60 years, with a male:female ratio of 1.6:2 (1). The duration from onset to diagnosis is in the range 4–6 years (2). Staging of MF and Sézary syndrome (SS) is based on a tumour node metastasis (TNM) classification system (1997, 2007), which is used for stratification of treatment recommendations.
The choice of treatment depends on the type of PCL and the stage of the disease. Recommendations are based on consensus by lymphoma societies (3, 4). The 3 most common skin treatments for early-stage MF are topical steroids, phototherapy, and topical chlormethine (also known as mechlorethamine), a bifunctional cytotoxic alkylating agent that results in cell death (apoptosis). In 1950, aqueous chlormethine (Caryolysine) received initial approval in the USA for topical treatment of MF (and was also used in Europe). In the early 1980s, chlormethine compounded in Aquaphor (a petroleum-based ointment) was introduced. All compounded forms had the disadvantage of instability and lack of reproducibility of results.
A novel topical chlormethine (CL) gel was developed in 2004, and was approved for use in the USA and EU in 2013 and 2017, respectively, based on a randomized controlled trial 201 (5), in which the efficacy and safety of 0.02% CL gel were found to be non-inferior to 0.02% compounded ointment (5). The overall response to treatment with 0.02% CL gel (59%) was greater than with the compounded 0.02% CL ointment (48%). In study 201 (5), 20% of enrolled patients treated with CL gel and 17% treated with CL ointment withdrew due to drug-related skin irritation. Among the adverse events (AEs) reported, dermatitis was the main reason for discontinuing treatment. The use of steroids was not allowed within the registration trial, but concomitant use of corticosteroids with CL gel is currently being studied, with the aim of better understanding the potential use of corticosteroids for management of such skin reactions. A number of case studies and clinical practice experiences have been published, highlighting that, in the real-world setting, CL gel is well-tolerated, with treatment-emergent AEs generally mild in nature and effectively managed with appropriate topical interventions and dosing modifications (6, 7).
The aim of this study was to evaluate the real-life efficacy and side-effect profile of CL gel in patients with MF
PATIENTS AND METHODS
Patients with different types of classic MF skin lesions (patches, plaques, tumours) were treated with CL gel at the National Center of excellence for the treatment of CL, Attikon University Hospital, Athens, Greece, between September 2020 and May 2021. All patients had a histologically confirmed diagnosis of MF, except for 1 patient with gamma-delta epidermotropic PCL, which was diagnosed as MF on clinical grounds.
All patients were evaluated at baseline, and at months 1, 3, 6 and 9, for efficacy and AEs. Overall response was recorded using the modified Severity Weighted Assessment Tool (mSWAT) score, time to response (TR), and time to next treatment (TNT).
Patients were instructed to use the CL gel at night. In case of dermatitis, a dose reduction to 3 times per week was advised, in addition to emollient creams. In cases of severe dermatitis, use of clobetasol and temporary discontinuation of CL gel was advised. Patients were instructed to continue with treatment with close-monitoring and frequent visits.
RESULTS
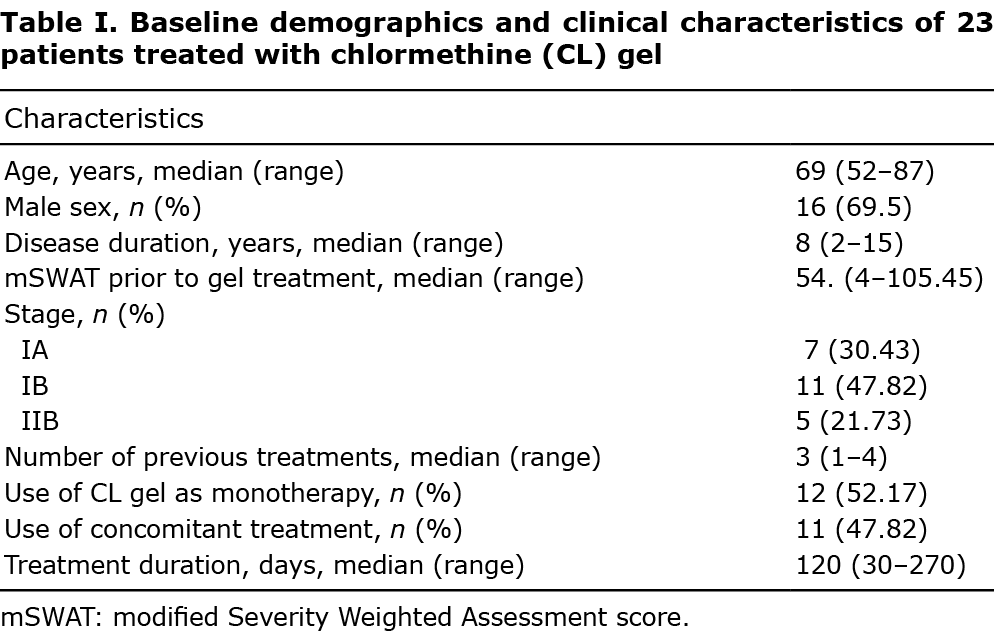
The baseline demographic and clinical characteristics of patients treated with CL gel are shown in Table I. Twenty- three patients, 16 men and 7 women (male:female ratio 2.3:1), median age 69.5 years (range 52–87) were treated. Median duration of disease was 8 years (range 2–15 years). Before gel application, patients had a median mSWAT of 54.73 (range 4–105.45) and 18/23 (78.26%) were early-stage (7/23 stage IA, 11/23 stage IB), and 5/ 23 (21.73%) advanced-stage IIB MF. CL gel was given as monotherapy in 12/23 patients, and in combination with methotrexate (MTX) or peginterferon alpha-2a in 11/23 patients.
The results for efficacy and AEs are shown in Table II. At month 1, all patients had stable disease (SD). At month 3, 10/23 patients showed partial response (PR) with mean reduction in mSWAT 60% from baseline, while 8/23 patients remained at SD. At month 6, 13/23 patients showed overall response, with 5/13 near complete response (NRCR, 90% mSWAT improvement) and 8/13 with PR. At month 9, 15/23 patients achieved overall response; 5/15 showed CR with mSWAT 0, 2/15 NRCR, and 8/15 showed PR with reduction in mSWAT of 75%.

Overall response in stages IB/IIB was delayed compared with IA (this result was not statistically significant due to the small number of patients, and remains to be clarified in future studies).
At month 1 of gel treatment, AEs were observed in 10/23 patients with MF and at month 3 these had resolved (Table II). Mild dermatitis was observed in 7/10 patients, and 3/23 patients developed severe dermatitis at month 1, leading to ulceration in 2/3 patients at month 3 with high overall response at months 6 or 9 (2 of these achieved PR and 1 CR). All patients continued treatment with CL gel with resolution of AEs at months 6 and 9. No skin malignancy was observed during treatment with CL gel.
One stage IIB patient died due to respiratory infection, which was not related to the treatment, 3 discontinued treatment in the first month due to dermatitis that could not be tolerated, and 1 patient with gamma-delta epidermotropic MF discontinued treatment due to disease progression.
DISCUSSION
This study of a small group of patients found that CL gel is a well-tolerated and safe treatment for MF lesions, such as patches, plaques and tumours. A better and more rapid response was observed in patients with early and limited disease (IA). All patients with MF treated with CL gel achieved stable disease at month 1, and at month 3 half of the patients achieved PR. Despite close monitoring 3 out of 7 stage IA patients discontinued treatment in the first month due to dermatitis. In order achieve better compliance the frequency of application of CL gel was reduced to 3 times per week. At month 3, all stage IA patients who continued the gel achieved PR, while at month 9 all achieved CR. Of the 11 stage IB patients, apart from 1 patient with gamma-delta MF who discontinued treatment at month 1 due to skin progression, at month 3 5/10 patients, at month 6 6/10, and at month 9, six patients achieved PR and 2/10 achieved CR. One out of 4 stage IIB patients achieved NRCR and 2 achieved PR from month 3. All stage IIB patients were on systemic treatment, and achieved clinical response when CL gel was added to treat a few localized tumour lesions refractory to systemic treatment. Notably, 1 stage IIB patient on peginterferon alpha-2a with partial response achieved CR at month 9 of combination treatment with CL gel.
To the best of our knowledge, this is the first study to use CL gel in all clinical MF lesions including tumours, which were not included in study 201(5). The response rates at all months were similar to those in study 201 (5). CL gel was well tolerated, with good clinical outcomes reported from month 1. Since CL gel was available in June 2019 in Greece, some of the current study patients had not yet reached 1-year treatment, and peak clinical response among the stage IA–IIB patients was higher at month 9 (65.22%). In the PROVE study, which has a longer follow-up, peak response was seen later (at 18 months, 66.7%) among stage IA–IB patients (6).
CL gel was generally well tolerated, except for in 10 patients who developed dermatitis. Throughout the current study 43.47% of patients developed some degree of dermatitis, 13.04% developed medium-to-severe dermatitis during the first month of treatment, but only 3 patients using CL gel discontinued treatment due to dermatitis. Based on results from clinical practice, the management of gel application was adjusted from daily to 3 times per week, which led to better patient compliance. Management of patients with severe dermatitis was through close monitoring and down-tapering of gel use to 3 times per week and use of potent topical steroids (Table III). It was observed that the presence of dermatitis did not have an impact on the efficacy of CL gel. More specifically, 3 patients with severe acute dermatitis finally achieved overall response. In the PROVE study, among 298 adult patients with MF-CLTF, dermatitis/skin irritation rates (12.8%/7.4%, respectively) were lower than observed in study 201, possibly due to concomitant steroid treatment and/or dosing modifications (8). In the current study no malignancy was observed at gel application sites at 9-month follow-up. In the MIDAS study, which is investigating the incidence and severity of contact dermatitis following treatment with CL gel in patients MF-CTCL stages IA–IB, mild-to-moderate dermatitis may not require suspension of treatment, but may require emollients or topical steroids or decreased dosing frequency (7, 9) (Table IV).


In our experience, close monitoring is very important in order to maintain patients on treatment. We monitor patients closely throughout treatment, especially during the first months when AEs usually occur. During the national lockdown due to the COVID-19 pandemic, we continued to monitor most of our patients via teleconferencing, urging them not to stop treatment. In 5 patients with difficulty getting the treatment on time, a relapse in skin disease, but not progression, was observed. In the meantime, patients were instructed to continue treatment with clobetasol propionate. After the end of national lockdown, approximately 3 months, patients restarted treatment with CL gel with no recurrence of AEs. In a large study of 4,922 patients, it was shown that, in settings with higher patient volume, patients sustained longer treatment duration and, importantly, avoided early discontinuation due to better management of the disease and dermatitis (10). In the current study, only 4 patients discontinued treatment, which is attributed to our increasing experience and good physician–patient inter-relationship.
The current study has some limitations. First, the chronic period of CL gel use was short due to the availability of CL gel in Greece. However, our last follow-up at 12 months has not shown significant differences. From the 15 patients with overall response at month 9, 2/15 (13.3%) patients achieved CR at month 12, 8/15 (53.3%) patients achieved PR at month 12, and 5/15 (33.3%) patients completed treatment with CL gel, maintaining the response for at least 1 month after treatment. Finally, in patients who had to discontinue treatment, the study did not perform a patch test in order to differentiate allergic reactions from irritant reactions.
Both physicians and patients should be educated in the management of MF with CL gel treatment and close monitoring, in order to avoid premature discontinuation and loss of compliance. Continuous use of CL gel treatment is required in order to achieve maximum response. Larger studies with more data are important in order to assess the use of CL gel in patients with different MF lesions.
The authors have no conflicts of interest to declare.
REFERENCES
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713–1722.
- Scarisbrick JJ, Quaglino P, Prince HM, Papadavid E, Hodak E, Bagot M, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol 2019; 181: 350–357.
- Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome – update 2017. Eur J Cancer 2017; 77: 57–74.
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology (NCCN Guidelines®) cervical cancer. [accessed 2021 Dec 1]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol 2013; 149: 25–32.
- Kim EJ, Geskin L, Guitart J, Querfeld C, Girardi M, Musiek A, et al. Real-world experience with mechlorethamine gel in patients with mycosis fungoides-cutaneous lymphoma: preliminary findings from a prospective observational study. J Am Acad Dermatol 2020; 83: 928–930.
- Gilmore ES, Alexander-Savino CV, Chung CG, Poligone B. Evaluation and management of patients with early-stage mycosis fungoides who interrupt or discontinue topical mechlorethamine gel because of dermatitis. JAAD Case Rep 2020; 6: 878–881.
- Kim EJ, Guitart J, Querfeld C, Girardi M, Musiek A, Akilov OE, et al. The PROVe study: US real-world experience with chlormethine/mechlorethamine gel in combination with other therapies for patients with mycosis fungoides cutaneous T-cell lymphoma. Am J Clin Dermatol 2021; 22: 407–414.
- Gilmore ES, Alexander-Savino CV, Chung CG, Poligone B. Incidence and types of contact dermatitis after chlormethine gel treatment in patients with mycosis fungoides-type cutaneous T-cell lymphoma: the MIDAS study. In: 4th World Congress of Cutaneous Lymphomas; 2020 Feb 12–14; Barcelona, Spain. WCCL, ISCL; 2020. Abstract number Y-04. [accessed 2021 Dec 1] Available from: http://wcclbarcelona2020.com/images/site/PROTEGIDO/Abstracts_WCCl_2020_DIGITAL.pdf.
- Querfeld C, Pacheco T, Haverkos B. Binder G, Angello J, Poligone B. Treatment duration as a function of clinician-level patient volume: mechlorethamine treatment for mycosis fungoides cutaneous T-cell lymphoma (MF-CTCL). [accessed 2021 Dec 1] Available from: https://www.oncnet.com/meeting-materials/lymphoma-and-myeloma/4787.