Pyogenic granuloma is one of the most common vascular tumours. The cause of pyogenic granuloma was previously thought to be an inflammatory reaction with consecutive stimulation of endothelial cell proliferation. However, recent studies suggest that pyogenic granuloma may be driven by constitutive activation of the mitogen-activated protein kinase pathway. The aim of this study was to investigate the molecular profile of sporadic pyogenic granuloma of childhood, using a systematic approach scrutinizing potential aberrations within different oncogenic pathways. Within a retrospective setting pyogenic granuloma of 15 patients was analysed by targeted next generation sequencing using the Oncomine Focus Assay, which includes genes of key tumorigenic signalling pathways. Activating mutations were found in 4 out of 15 cases (27%). Two HRAS hotspot mutations (p.Gly13Arg, p.Ala59Thr), 1 BRAF (p.Val600Glu) mutation and a novel, previously not reported, MAP2K1 hotspot mutation (p.Glu203Lys) were identified. It is notable that all of these genes are involved in constitutive mitogen- activated protein kinase signalling. This study increases the range of underlying genetic alterations in pyogenic granuloma by identifying novel oncogenic mutations in crucial mitogen-activated protein kinase pathway genes. The results provide supporting evidence that activated mitogen-activated protein kinase signalling is a key driver in the pathogenesis of pyogenic granuloma, which might be exploited by targeted treatment approaches for selected cases.
Key words: pyogenic granuloma; targeted sequencing; MAPK pathway.
Accepted Apr 8, 2022; Epub ahead of print Apr 8, 2022
Acta Derm Venereol 2022; 102: adv00715.
DOI: 10.2340/actadv.v102.1119
Corr: Marion Wobser, Department of Dermatology, University Hospital Würzburg, Josef-Schneider-Str. 2, DE-97080 Würzburg, Germany. E-mail: Wobser_M@ukw.de
SIGNIFICANCE
Pyogenic granuloma is a common vascular tumour. This retrospective study analysed sporadic pyogenic granuloma of 15 children, using targeted next generation sequencing of genes implicated in key tumorigenic signalling pathways. In 27% of analysed cases alterations in genes of the mitogen-activated protein kinase pathway were identified, thereby implying that constitutive mitogen-activated protein kinase signalling plays a critical role in the pathogenesis of sporadic PG. Hence, the previous concept that pyogenic granuloma reflects a reactive endothelial cell proliferation due to inflammatory triggers is revised. Despite somatic alterations in oncogenes, the biological nature of pyogenic granuloma is benign, implying mechanisms such as oncogene-induced senescence.
INTRODUCTION
Vascular anomalies (vascular tumours and vascular malformations) represent a broad spectrum of disorders with diverse biological behaviour and distinct clinical presentation (1). Evidence to support a more precise classification has grown in recent years, especially based on more sophisticated genetic analyses and, thus, deeper molecular insight.
Pyogenic granuloma (PG) is one of the most common vascular tumours. It shows a benign behaviour and may affect both skin and mucous membranes. Clinically, PG usually presents as a solitary, red, exophytic papule. Iterative bleeding is the most common symptom leading to consultation with a physician. In some cases, satellite lesions are present (agminated PG) or disseminated lesions are rare. PG is commonly located in the head and neck area.
PG was previously thought to be caused by an inflammatory (eponymously “granulomatous”) reaction to an infectious or traumatic event, leading to the term “pyogenic granuloma” (2). However, in recent years, deeper insight into the molecular pathogenesis of PG has been gained. Based on these findings, the previously assumed putative reactive origin of PG has been debated. As a consequence, the term “lobular capillary haemangioma” has been proposed to better classify this vascular tumour. PGs have been observed to arise occasionally in association with vascular malformations, such as a port-wine stain (PWS) (3), but in most cases they occur spontaneously without a syndromic context.
PWS commonly exhibit activating mutations in GNAQ, occurring in more than 90% of cases (4). Recently, it has been shown that PG arising within the context of a PWS share similar GNAQ mutations with the underlying PWS. However, in PWS-associated PG further somatic single nucleotide variations (SNV) are present, distinguishing both vascular lesions at a molecular level. In a mutually exclusive pattern both oncogenic BRAF mutations (BRAF c.1799T>A (p.Val600Glu)) as well as (albeit at lower frequencies) RAS mutations (NRAS c.182A>G (p.Q61R) were detected in PWS-associated PG. The underlying PWS revealed wild-type status for both BRAF or NRAS in all analysed cases. Likewise, sporadic PG of adults and children (occurring without the context of PWS) have been shown to harbour different BRAF, KRAS, NRAS or HRAS mutations with variable frequencies within the different studies (5, 6). In line with these molecular data, Pereira et al. demonstrated activation of the mitogen-activated protein kinase (MAPK) pathway on a more functional level by immunohistochemically detecting phosphorylation of the downstream targets ERK1/2 in endothelial cells (7). Taken together, these findings indicate that PG is a true neoplastic condition, which may be driven by constitutive activation of the MAPK pathway on the ground of oncogenic somatic mutations.
Motivated by these observations this study investigated the molecular profile of sporadic PG of childhood in more detail by taking advantage of targeted next generation sequencing using the Oncomine Focus Assay (Thermo Fisher Scientific, Darmstadt, Germany). This approach enables investigation of the presence of pathogenic activating mutations within cardinal key oncogenic signalling components including, but not restricted to, the MAPK pathway.
MATERIALS AND METHODS
Patient characteristics
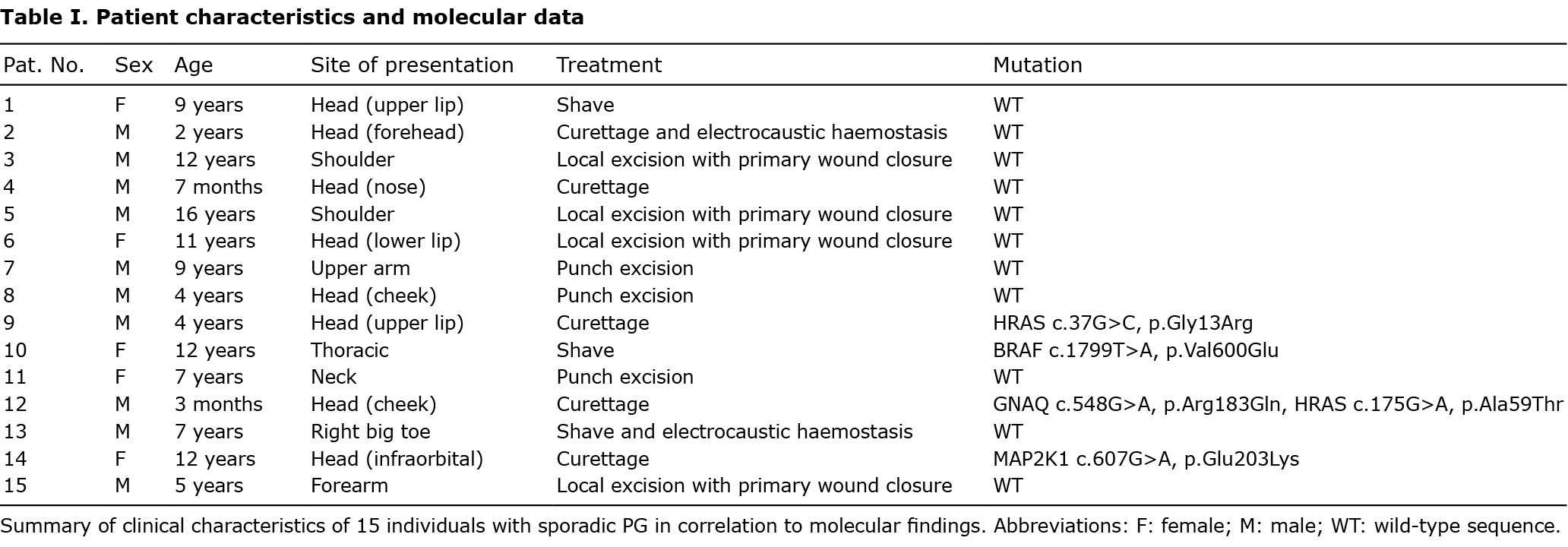
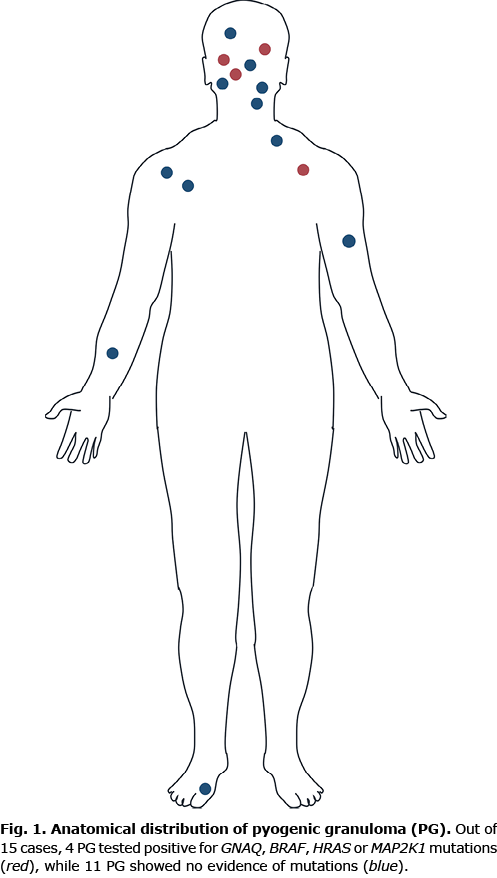
Fifteen patients with sporadic PGs, age range 3 months to 16 years, mean age 6.7 years, were retrospectively identified in the clinical and histological files of the Department of Dermatology, University Hospital Würzburg, Germany. All patients were diagnosed with PG in close clinicopathological correlation. The male to female ratio was 3:1. The most frequently affected site was the head/neck area (9 out of 15). All patients underwent surgery (excision, curettage or shave) under local anaesthesia achieving a complete remission. Further patient details are shown in Table I. An overview of the anatomical distribution of respective lesions is shown in Fig. 1. Representative clinical images are shown in Fig. 2. Study approval was obtained from the ethics committee at the Medical Faculty of the University of Würzburg, Germany, in compliance with the Declaration of Helsinki. Informed written consent was obtained from patients or their parents prior to analysis. Patients/parents also consented to publication of their images.
DNA extraction
Genomic DNA was extracted from formalin-fixed, paraffin-embedded sections after microdissection, using the Maxwell RSC Blood DNA Kit (Promega GmbH, Walldorf, Germany). After pre-treatment with a THG1-thioglycerol/incubation buffer mix for 10 min at 80°C and subsequent incubation with proteinase K at 65°C overnight (Promega GmbH, Walldorf, Germany). DNA quantity and quality was assessed by quantitative PCR (TaqMan RNase P Detection Reagents Kit, Life Technologies, Darmstadt, Germany).
Multiplex PCR-based panel sequencing
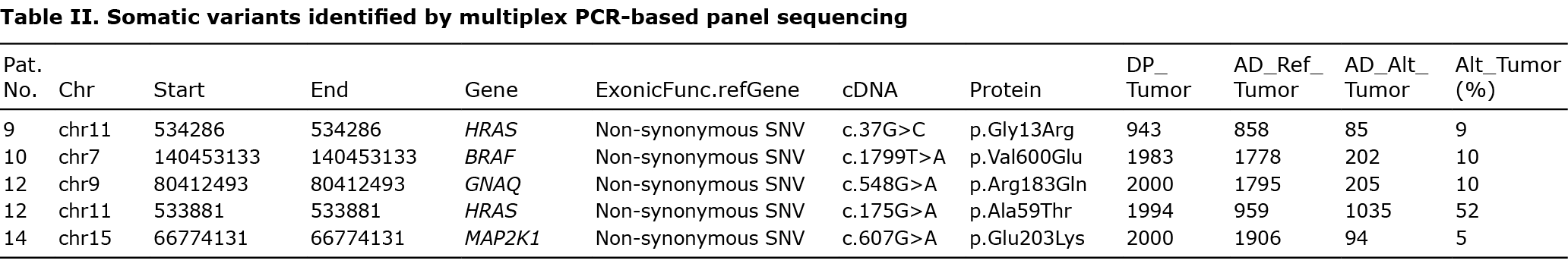
DNA libraries were prepared with the DNA part of the Oncomine Focus Assay and the Ion AmpliSeq Library Kit 2.0, according to the manufacturer’s recommendations. Subsequently, libraries were templated and enriched with the Ion OneTouch 2 and the Ion One Touch ES automated systems. Sequencing was performed using semiconductor sequencing technology (Ion Genestudio S5, Thermo Fisher Scientific). Data analysis was performed using the Torrent Server Variant Caller (v 5.10) and the Ion Reporter Software (v 5.12) (Thermo Fisher Scientific, Darmstadt, Germany). Samples were sequenced with a mean depth of 2,922×. Data were filtered for non-synonymous, exonic variants, and splice site variants in the flanking regions, showing an allele frequency of more than 2%. Variants in the general reported population with a minor allele frequency of more than 0.5% in 1,000 Genomes and dbSNP were excluded (Table II).
RESULTS
Panel sequencing shows recurrent activating mutations in genes of the mitogen-activated protein kinase signalling pathway
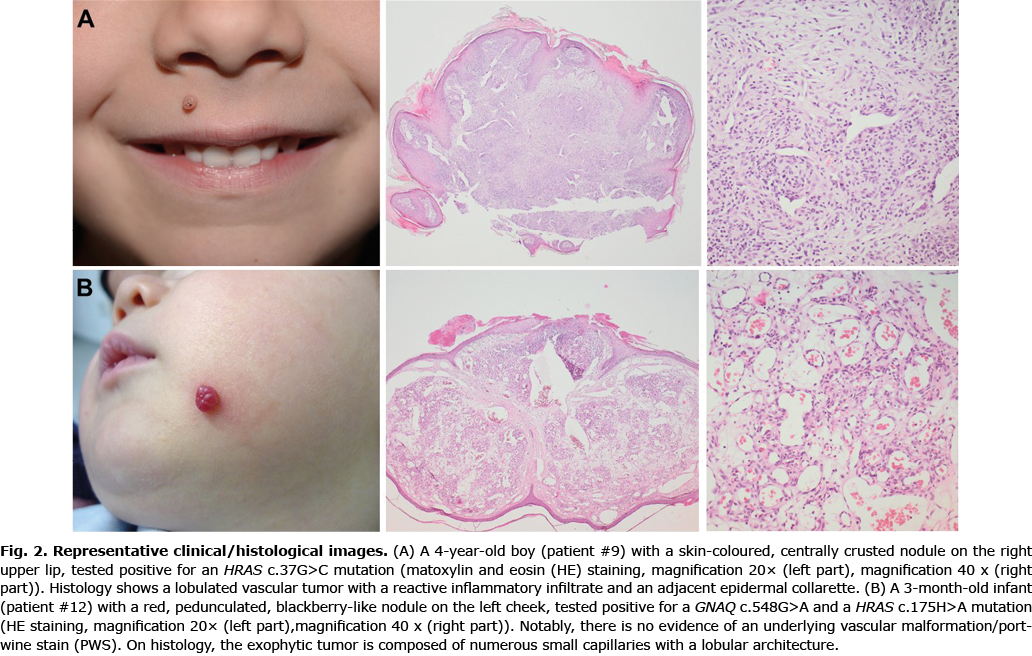
To decipher the pathogenesis of PG and identify crucial molecular driver mechanisms, explorative targeted next generation sequencing was performed using the Oncomine Focus Assay, which addresses activating genetic alterations within key oncogenic signalling pathways. This approach includes > 50 genes coding for different receptor tyrosine kinases and further pivotal components of well-described oncogenic driver pathways, including the phosphatidylinositol 3-kinase (PI3K) and MAPK signalling pathway. Fifteen samples from children and adolescents with sporadic PG were sequenced. The anatomical localization of sporadic PGs with (n = 4) and without (n = 11) mutations is shown in Fig. 1. Representative clinical and histological findings of selected mutated cases are shown in Fig. 2. Table I summarizes clinical characteristics of these 15 individuals in correlation to molecular findings.
Within this cohort, 5 mutations were detected in 4 patients (4 out of 15). Two HRAS mutations were identified, affecting codons 13 and 59 (p.Gly13Arg, p.Ala59Thr) in 2 patients (patients #9 and #12). Moreover, a BRAF V600E (p.Val600Glu) mutation (patient #10) and a MAP2K1 mutation in codon 203 (p.Glu203Lys) became apparent (patient #14). Of note, patient #12 showed, in addition to a HRAS (p.Ala59Thr) mutation, an activating GNAQ hotspot mutation (p.Arg183Gln). This patient was a 3-month-old child with a sporadic PG on the cheek, which was not associated with a PWS phenotype (Fig. 2B). No difference was observed on clinical or histological grounds between mutated or unmutated cases, in that all the cases of PG showed complete remission after removal.
DISCUSSION
In recent years significant progress has been made in elucidating the molecular pathogenesis of vascular malformations and vascular tumours. In various benign and malignant proliferations of endothelial cells several cardinal signalling pathways have been identified as being altered, especially via somatic mutations. Most of these genetic alterations are not subtype-specific. Similar hotspot mutations are widely distributed among different vascular disorders, somewhat irrespective of biological behaviour. Constitutive downstream signalling affects multiple biological functions, such as cellular proliferation, differentiation, apoptosis, metabolism, cytoskeletal arrangement and vasopermeability of endothelial cells (8, 9).
Recent studies suggest that PG is not a reactive process, but a true neoplastic condition possibly driven by constitutive activation of the MAPK pathway due to somatic mutations (10).
Genes of the guanine nucleotide-binding protein subunit alpha q (Gαq) family (GNAQ, GNA11, GNA14) represent upstream activators of different pathways, including the phospholipase C (PLC) pathway, the PI3K-Akt-mTOR pathway, and the MAPK signalling cascade. Gαq proteins have been shown to mediate vascular endothelial growth factor- (VEGF-) dependent migration and proliferation of endothelial cells in vitro (11). On this basis, Shirley et al. reported that somatic mutations in the GNAQ gene (p.Arg183Gln) in PWS blood vessels resulted in activation of downstream MAPK signalling (4). Another study suggested that GNA14 and GNA11 mutations in kaposiform haemangioendotheliomas and congenital tufted angiomas induce changes in cellular morphology and growth factor independence via MAPK activation (12). PG arising within the context of a PWS share similar GNAQ mutations with the underlying PWS (5) in addition to further driver mutations of the MAPK pathway being exclusively present in PG.
We describe here the novel finding of a GNAQ hotspot mutation (p.Arg183Gln) that occurred in addition to an oncogenic HRAS mutation (p.Ala59Thr) in sporadic PG not being associated with a PWS phenotype (13, 14). Taken together the previous and current findings raise the hypothesis that, in sporadic PG also, a second hit (in MAPK genes) in addition to a mutation in components of the Gαq family of genes is necessary to drive the full phenotype (5). In fact, in vascular malformations, such as PWS, only slow progression is observed, whereas vascular tumours, such as PG, proliferate rapidly despite analogous activating GNAQ mutations (2, 4). This is in line with the fact that cherry haemangioma invariably displays an indolent behaviour despite frequent (>80%) presence of GNA14, GNAQ and GNA11 mutations without, however, further single nucleotide variations (SNVs) (15). The oncogenic properties of GNAQ mutations in combination with further tumour-promoting events are also evidenced in non-vascular tumours, e.g. uveal melanoma, a metastasis-prone, highly malignant tumour exhibiting recurrent SNVs in genes of the G protein family in conjunction with further genetic aberrations (16, 17).
In 27% of the analysed cases in the current study activating mutations in genes of the MAPK pathway were identified, while mutations in other signalling pathways could not be identified. Although oncogenic mutations were identified in different genes, respective constitutive downstream signalling converges within the same MAPK signalling cascade and, thus, finally drives similar functional biological consequences. In order to elucidate whether further oncogenic pathways beyond the MAPK cascade might be involved in the molecular pathogenesis of PG we applied the Oncomine Focus Assay, which comprises a wide spectrum of different pathway-related genes. However, all of these other genes showed wildtype sequences. This emphasizes that activation of the MAPK pathway at different stages of the signalling cascade represents the major driving force of PG. As this methodological approach focussed only on a selected set of genes, including the most common hot-spot mutations in MAPK genes, we cannot exclude that further genetic alterations activating this pathway might be present in the remaining negative cases in the current study cohort, which are missed by the current analysis. It is notable that 1 of the mutations identified herein, i.e. an oncogenic MAP2K1 mutation (18, 19), has not been described previously in PG, but also fits into the concept of an activated MAPK pathway.
Driver mutations in RAS (mainly KRAS) are the most common mutations in cancer, occurring in approximately 30% of all cancer types (20). RAS mutations are attributed to have a proangiogenic effect on endothelial cells (21). Moreover, RAF mutations (particularly in BRAF) are present in approximately 8% of all cancer types (22). Although genetic aberrations of these same genes have been shown to orchestrate a malignant phenotype in distinct vascular malignant tumours, such as angiosarcoma (23), albeit at lower frequencies, PG does not develop any malignant properties despite these genetic alterations. The underlying mechanism for this observation is hitherto unknown, but may rely on features of oncogene-induced senescence (24).
To date, no mutations in the MAPK pathway have been detected in either periocular or oral/mucosal PGs. We have now identified an activating MAP2K1 hotspot mutation in a PG located infra-orbitally. The fact that, altogether, in only 20–30% of cases of PG single nucleotide variations (SNVs) could be detected in different studies, may be due to limited patient number (as was also the case in the current analysis) or the variable methodological approaches including single gene assays (7, 25). Therefore, the current findings highlight the advantage of a high throughput sequencing approach, which allows a sensitive and simultaneous analysis of plenty of relevant driver genes involving various signalling pathways prior to single gene analyses.
To summarize, these data extend the spectrum of somatic mutations in PG. The results of this study strengthen the evidence that constitutive MAPK signalling by activating mutations plays a critical role in the pathogenesis of sporadic PG.
ACKNOWLEDGEMENTS
We thank all patients for participating in this study. The patients in this manuscript have given written informed consent to publication of their case details. We are grateful to all of our colleagues for the histopathological work-up of biopsy specimens and clinical management of the patients.
Data will be provided by the corresponding author upon request for reasonable academic studies.
The authors have no conflicts of interest to declare.
REFERENCES
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015; 136: e203–e214.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol 1991; 8: 267–276.
- Chen D, Hu XJ, Lin XX, Ma G, Jin YB, Chen H, et al. Nodules arising within port-wine stains: a clinicopathologic study of 31 cases. Am J Dermatopathol 2011; 33: 144–151.
- Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge–Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med 2013; 368: 1971–1979.
- Groesser L, Peterhof E, Evert M, Landthaler M, Berneburg M, Hafner C. BRAF and RAS mutations in sporadic and secondary pyogenic granuloma. J Invest Dermatol 2016; 136: 481–486.
- Lim YH, Douglas SR, Ko CJ, Antaya RJ, McNiff JM, Zhou J, et al. Somatic activating RAS mutations cause vascular tumors including pyogenic granuloma. J Invest Dermatol 2015; 135: 1698–1700.
- Pereira T, de Amorim LSD, Pereira NB, Vitório JG, Duarte-Andrade FF, Guimarães LM, et al. Oral pyogenic granulomas show MAPK/ERK signaling pathway activation, which occurs independently of BRAF, KRAS, HRAS, NRAS, GNA11, and GNA14 mutations. J Oral Pathol Med 2019; 48: 906–910.
- Nguyen V, Hochman M, Mihm MC, Jr., Nelson JS, Tan W. The pathogenesis of port wine stain and Sturge Weber syndrome: complex interactions between genetic alterations and aberrant MAPK and PI3K activation. Int J Mol Sci 2019; 20: 2243.
- Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011; 4: 51.
- Cheraghlou S, Lim Y, Choate K. Genetic investigation of childhood vascular tumor biology reveals pathways for therapeutic intervention. F1000Res 2019; 8: 590.
- Zeng H, Zhao D, Yang S, Datta K, Mukhopadhyay D. Heterotrimeric G alpha q/G alpha 11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J Biol Chem 2003; 278: 20738–20745.
- Lim YH, Bacchiocchi A, Qiu J, Straub R, Bruckner A, Bercovitch L, et al. GNA14 somatic mutation causes congenital and sporadic vascular tumors by MAPK activation. Am J Hum Genet 2016; 99: 443–450.
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017; 23: 703–713.
- Lacal JC, Srivastava SK, Anderson PS, Aaronson SA. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell 1986; 44: 609–617.
- Liau JY, Lee JC, Tsai JH, Chen CC, Chung YC, Wang YH. High frequency of GNA14, GNAQ, and GNA11 mutations in cherry hemangioma: a histopathological and molecular study of 85 cases indicating GNA14 as the most commonly mutated gene in vascular neoplasms. Mod Pathol 2019; 32: 1657–1665.
- Patel M, Smyth E, Chapman PB, Wolchok JD, Schwartz GK, Abramson DH, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin Cancer Res 2011; 17: 2087–2100.
- Li Y, Shi J, Yang J, Ge S, Zhang J, Jia R, et al. Uveal melanoma: progress in molecular biology and therapeutics. Ther Adv Med Oncol 2020; 12: 1758835920965852.
- Gao Y, Chang MT, McKay D, Na N, Zhou B, Yaeger R, et al. Allele-specific mechanisms of activation of MEK1 mutants determine their properties. Cancer Discov 2018; 8: 648–661.
- Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C, et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat Genet 2011; 44: 133–139.
- Maik-Rachline G, Hacohen-Lev-Ran A, Seger R. Nuclear ERK: mechanism of translocation, substrates, and role in cancer. Int J Mol Sci 2019; 20: 1194.
- Meadows KN, Bryant P, Vincent PA, Pumiglia KM. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene 2004; 23: 192–200.
- Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell 2018; 173: 321–37.e10.
- Jansen P, Müller H, Lodde GC, Zaremba A, Möller I, Sucker A, et al. GNA14, GNA11, and GNAQ mutations are frequent in benign but not malignant cutaneous vascular tumors. Front Genet 2021; 12: 663272.
- Zhu H, Blake S, Kusuma FK, Pearson RB, Kang J, Chan KT. Oncogene-induced senescence: from biology to therapy. Mech Ageing Dev 2020; 187: 111229.
- Li G, Adams E, Eshleman JR, Eberhart CG. No BRAF V600E mutation identified in 28 periocular pyogenic granuloma. Ophthalmic Plast Reconstr Surg 2018; 34: 525–527.