This study identified risk factors for the development of dupilumab-associated ocular surface disease in patients with moderate-to-severe atopic dermatitis in a large prospective daily practice cohort. Data from the Dutch BioDay Registry were used to assess the risk of developing dupilumab-associated ocular surface disease, by performing univariate and multivariate logistic regression analyses. A total of 469 patients were included, of which 152/469 (32.4%) developed dupilumab-associated ocular surface disease. Multivariate analysis showed a statistically significant association of the development of dupilumab-associated ocular surface disease with a history of any eye disease (history of self-reported episodic acute allergic conjunctivitis excluded) combined with the use of ophthalmic medication at the start of dupilumab (odds ratio 5.16, 95% confidence interval 2.30–11.56, p < 0.001). In conclusion, a history of any eye disease (history of self-reported episodic acute allergic conjunctivitis excluded) combined with the use of ophthalmic medication at baseline was associated with the development of dupilumab-associated ocular surface disease in patients with atopic dermatitis.
Key words: conjunctivitis; dupilumab; dupilumab-associated ocular surface disease; DAOSD.
Accepted Dec 15, 2021; Epub ahead of print Jan 31, 2022
Acta Derm Venereol 2022; 102: adv00666.
DOI: 10.2340/actadv.v102.1128
Corr: Roselie Achten, Department of Dermatology and Allergology, University Medical Center Utrecht, Heidelberglaan 100, NL-3584 CX Utrecht, The Netherlands. E-mail: R.E.Achten@umcutrecht.nl
SIGNIFICANCE
Dupilumab-associated ocular surface disease is the most frequently reported side-effect during dupilumab treatment in patients with atopic dermatitis. Although risk factors have been studied, there is a lack of data from large prospective daily practice studies. This study found a significant association between the development of dupilumab-associated ocular surface disease and a history of any eye disease (history of self-reported episodic acute allergic conjunctivitis excluded) combined with the use of ophthalmic medication at the start of dupilumab treatment. Information about previous eye diseases and current ophthalmic medication can be helpful to assess the risk of the development of dupilumab-associated ocular surface disease.
INTRODUCTION
Dupilumab, a monoclonal antibody directed against the interleukin (IL)-4-receptor alpha subunit inhibiting both IL-4 and IL-13 signalling, is the first biologic agent for the treatment of patients with moderate-to-severe atopic dermatitis (AD). In multiple clinical trials and daily practice studies, dupilumab has proven to be effective with limited side-effects (1–6). Dupilumab significantly improves signs and symptoms of AD and increases the quality of life in patients with difficult-to-treat AD. Nevertheless, dupilumab-associated ocular surface disease (DAOSD), including conjunctivitis, eye pruritus, limbal nodules, and blepharoconjunctivitis developing during dupilumab treatment (7, 8), is the most frequently reported side-effect in patients with dupilumab-treated AD, and is reported in 9–34% in both clinical trials and daily practice studies (5, 9, 10). Remarkably, increased rates of DAOSD were not reported in dupilumab trials in other type-2 inflammatory diseases, such as asthma and chronic rhino sinusitis with nasal polyposis, suggesting that patients with AD may have a predisposition to develop DAOSD (9).
Currently, the pathomechanism of the development of DAOSD in patients with AD remains unclear. DAOSD and associated risk factors have been studied only in small cohorts and data from clinical trials (5, 9, 11–14). These studies suggested that the increased rates of DAOSD in patients with AD are associated with more severe AD at baseline, prior history of conjunctivitis, presence of other atopic comorbidities, eyelid involvement in AD, high levels of thymus and activation-regulated chemokine (TARC), high levels of serum IgE, and peripheral blood eosinophilia. However, prospective studies on risk factors for the development of DAOSD in patients with AD in daily practice are scarce.
Therefore, a prospective cohort study was conducted to identify risk factors for the development of DAOSD in a large group of patients with AD in daily practice.
MATERIALS AND METHODS
Study design and patients
This prospective multi-centre observational cohort study included patients with moderate-to-severe AD treated with dupilumab between October 2017 and February 2020 and registered in the Dutch prospective BioDay registry (5, 10). Patients were included from 8 different hospitals, including 3 academic hospitals. Included patients were aged ≥18 years and had a follow-up period for at least 16 weeks. This time-point was chosen, since most patients develop DAOSD within the first 16 weeks of dupilumab treatment (7, 9, 15). Patients who discontinued dupilumab within the first 16 weeks, but continued their follow-up for at least 16 weeks, were still included in the analyses.
At baseline, all patients received a 600 mg loading dose of dupilumab, followed by 300 mg injections every other week. Physicians diagnosed and reported the development of DAOSD by asking patients if they experienced symptoms of DAOSD, such as tearing, pruritus, or pain. In cooperation with the ophthalmologist, protocols were developed regarding the diagnosis and treatment of the DAOSD. There was no ophthalmological examination prior to the initiation of dupilumab. Only patients with difficult-to-treat DAOSD that could not be controlled with lubricant eye drops and/or tacrolimus skin ointment (1 mg/g) for the external eyelids, which was started after the onset of DAOSD, were referred to an ophthalmologist. The Medical Research Ethics Committee confirmed that this study did not fall under the scope of the Medical Research Involving Human Subjects act (METC 18/239). The study was performed according to the principles of the Declaration of Helsinki. All patients provided written informed consent.
Data collection
All data were extracted from the BioDay registry. Data collected at baseline included demographic information, medical history, physical examination, laboratory screening, and drug use (including previous and current ophthalmic drug use). Clinicians reported the medical history of any eye disease by asking patients about their general medical history and checking the electronical patient file. The medical history of any eye disease was divided into a medical history of allergic eye disease, and a medical history of non-allergic eye disease. A medical history of atopic keratoconjunctivitis, vernal keratoconjunctivitis, or giant papillary conjunctivitis was classified as a medical history of allergic eye disease. The medical history of non-allergic eye disease included keratoconus, pellucid marginal degeneration, keratitis, uveitis, herpetic keratitis, blepharitis, glaucoma, cataract, macula oedema, amblyopia, meibomitis, and retinal ablation. A history of self-reported episodic acute allergic conjunctivitis was reported separately, as this is usually not confirmed by an ophthalmologist. The severity of AD was assessed by the Eczema Area and Severity Index (EASI) and the Investigator’s Global Assessment (IGA). According to the Harmonising Outcome Measures for Eczema (HOME), EASI scores were classified as mild (0–5.9), moderate (6.0–22.9) and severe AD (23.0–72.0) (16). Patients being treated with oral immunosuppressive drugs at the time of initiation of dupilumab treatment were also classified as severe AD. EASI and laboratory results were collected at baseline, after 4 weeks, and after 16 weeks of treatment.
Statistical analyses
Patient characteristics were described using absolute numbers (N) and percentages for categorical variables, median with interquartile ranges (IQR) for non-normally distributed continuous variables, and mean with standard deviations (SD) for normally distributed continuous variables.
Univariate and multivariate logistic regression analyses were used to assess the contribution of potential risk factors to the development of DAOSD. Potential risk factors were selected based on (1) knowledge from previous literature, and (2) clinical experience. Risk factors based on knowledge from previous literature included severity of AD, eosinophils levels at baseline, presence of eyelid eczema, presence of other atopic conditions, and prior history of conjunctivitis (5, 9, 11–14). Since Thyssen (17) hypothesized that the development of DAOSD is related to the Demodex mites, which are elevated in rosacea, a history of rosacea was included as a variable. Another study by Thyssen et al. (18) concluded that the number of prescriptions of ophthalmic medication per person was higher in patients with AD compared with control subjects. In addition, Simonetti et al. (19) suggested that the use of moisturizing eye drops could be considered as a preventive resource for the development of DAOSD. For these reasons, the use of ophthalmic medication at baseline was included as a variable in our analyses.
Univariate analysis was performed on the variables as registered. In the multivariate analysis, some variables were combined, whilst others were either categorized or excluded. History of any eye disease was combined with the use of ophthalmic medication at baseline. AD severity categories based on EASI scores were included in the multivariate analyses, as these are widely used to define AD severity and have validated severity-ranges (16). In addition, AD eyelid involvement in the past year was excluded from the multivariate analyses due to a high number of missing values. No selection of risk factors was performed based on p-values from the univariate analyses.
The assumption of a linear association of continuous risk factors and the outcome was assessed with restricted cubic spline (20). Results were reported as odds ratio’s (OR) with 95% confidence intervals (95% CI) and p-values. p-values were adjusted for multiple testing using the Benjamini-Hochberg procedure (21), which controls false discovery rate (FDR). FDR adjusted p-values < 0.05 were considered statistically significant.
To investigate the possible association between clinical effectivness of dupilumab and the occurrence of DAOSD, changes in EASI scores were calculated between baseline and after 4 weeks of dupilumab treatment, and between baseline and after 16 weeks of dupilumab treatment. In addition, association between dupilumab-induced blood eosinophilia and the occurrence of DAOSD was studied. Comparison of the DAOSD group with the non-DAOSD group was conducted using the non-parametric Mann–Whitney U test. Changes in EASI scores and blood eosinophilia were not included in multivariate analyses, since these values reflect effectiveness of the treatment of dupilumab, and this study focussed on factors that may be assessed prior to start of the dupilumab.
Statistical analyses were conducted with SPSS Statistics version 25.0.0.2 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows) and the rms library package in R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and baseline characteristics
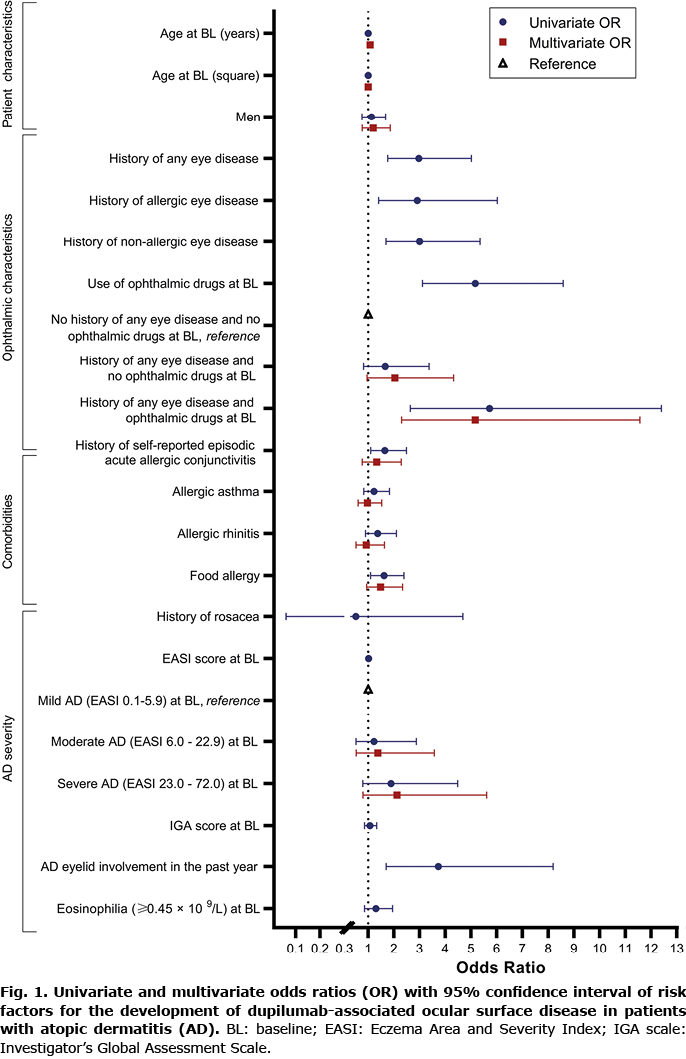
A total of 469 dupilumab-treated patients with AD were included at baseline with a median EASI score of 16.8 (IQR 10.9–26.1) and a median IGA score of 3 (IQR 3–4), shown in Table I. A medical history of any eye disease (excluding a history of self-reported episodic acute allergic conjunctivitis) was reported in 68/469 (14.5%) patients, and 81/469 (17.3%) patients were using ophthalmic drugs at the initiation of dupilumab treatment. A history of self-reported episodic acute allergic conjunctivitis was found in 274/469 (59.8%) patients. Within the first 16 weeks of dupilumab treatment, 11/469 (2.3%) patients discontinued their treatment. Three of these patients experienced ocular side-effects (2 patients with DAOSD and one patient with a cornea perforation). The other 8 patients stopped with dupilumab due to other reasons. All other baseline characteristics are shown in Table I.
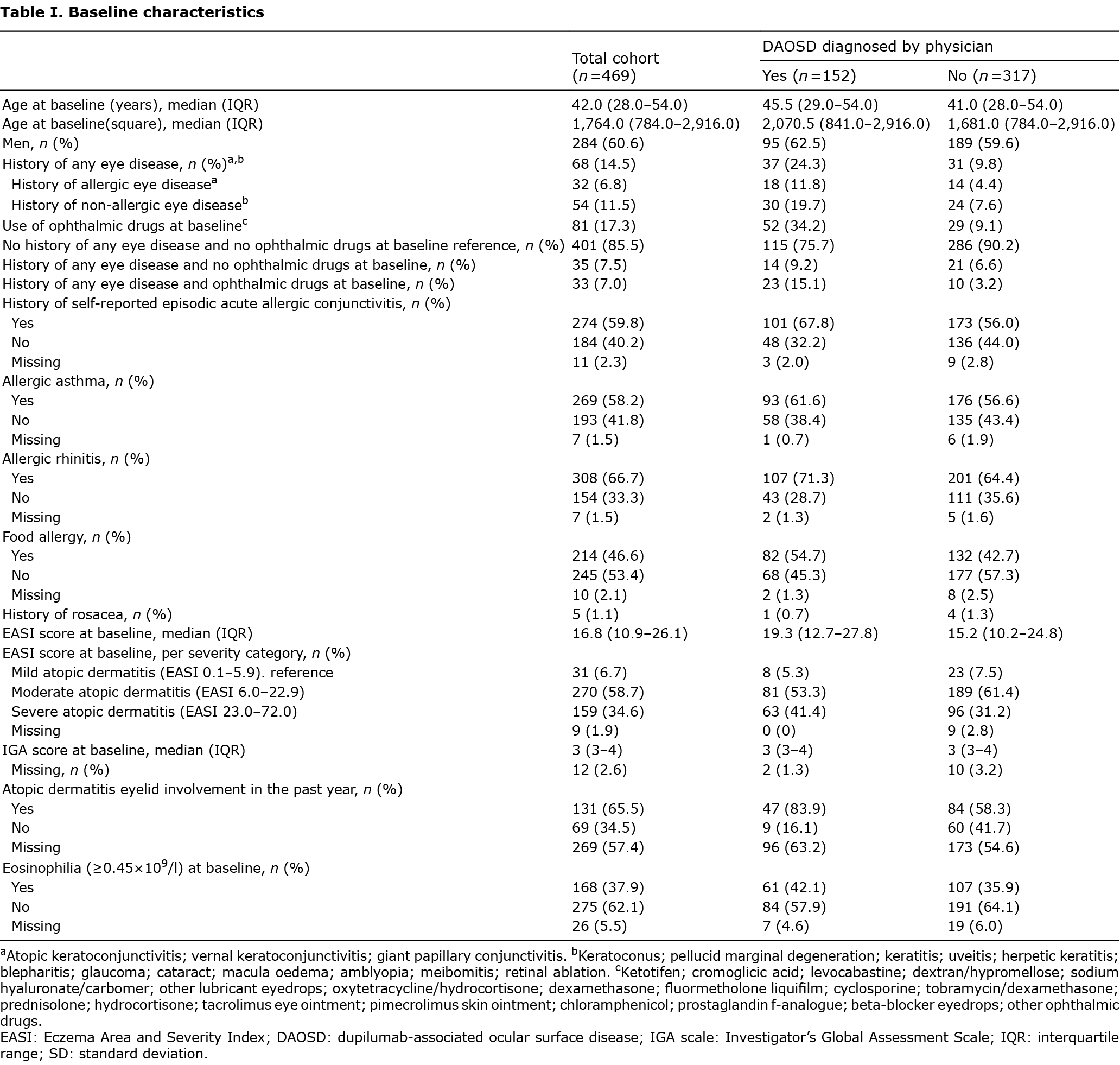
Development of dupilumab-associated ocular surface disease and univariate logistic regression odds ratios
Of all included patients, 152/469 (32.4%) developed DAOSD, as shown in Table I. Of these patients, 93/152 (61.2%) patients were referred to an ophthalmologist. Of all patients who developed DAOSD, 37/152 (24.3%) had a history of any eye disease, which was significantly higher compared with the patients who did not develop DAOSD (n = 31/317 (9.8%), OR 2.97, 95% CI 1.76–5.01, p < 0.001, Fig. 1, see Table SI). The number of patients who were using ophthalmic medication at baseline was significantly higher in the DAOSD group compared with the non-DAOSD group (OR 5.16, 95% CI 3.11–8.58, p < 0.001). Moreover, the number of patients with a history of any eye disease combined with the use of ophthalmic medication at baseline was significantly higher in the DAOSD group compared with the non-DAOSD group (OR 5.72 (95% CI 2.64–12.40, p < 0.001).
Furthermore, a history of self-reported episodic acute allergic conjunctivitis (OR 1.65, 95% CI 1.10–2.49, p = 0.039) and food allergy (OR 1.62, 95% CI 1.09–2.39, p = 0.039) were found to be significantly associated with the development of DAOSD.
Of the patients who developed DAOSD, more had AD on their eyelids in the past year, leading to a statistically significant association between eyelid involvement in AD in the past year and the development of DAOSD (OR 3.73, 95% CI 1.70–8.19, p = 0.004). Since this variable contained more than 100 missing values, it was excluded from the multivariate analyses.
EASI score at baseline showed no statistically significant association with the development of DAOSD (OR 1.02, 95% CI 1.00–1.03, p = 0.079).
Multivariate logistic regression odds ratios
Multivariate analysis showed a statistically significant association between having a history of any eye disease combined with the use of ophthalmic medication at baseline and the development of DAOSD (OR 5.16, 95% CI 2.30–11.56, p < 0.001, Fig. 1). No significant association was found between a history of self-reported episodic acute allergic conjunctivitis and the development of DAOSD in multivariate analysis (OR 1.33, 95% CI 0.77–2.29, p = 0.438).
With the exception of age, continuous risk factors showed a linear association with DAOSD development. Analysis with restricted cubic splines showed an association similar to a quadratic effect. For ease of interpretation, age and age-square were included in univariate and multivariate analysis.
Effectiveness of dupilumab and the development of dupilumab-associated ocular surface disease
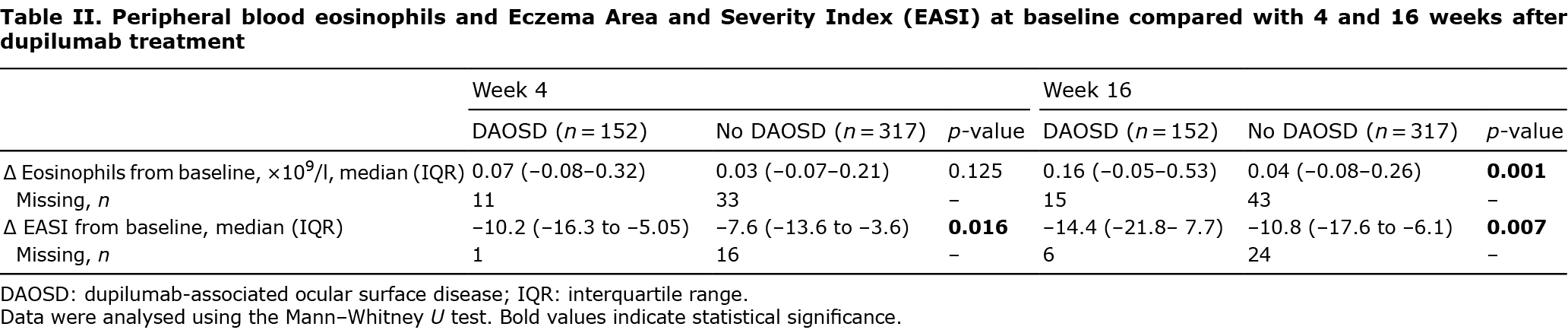
After 4 weeks of treatment with dupilumab, the decrease in EASI score from baseline was significantly higher in the DAOSD group, compared with the non- DAOSD group (p = 0.016, Table II). The significant difference in decrease continued during 16 weeks of treatment (p = 0.007).
Changes in peripheral blood eosinophils during dupilumab treatment
A significantly higher increase in peripheral blood eosinophils was found in the DAOSD group compared with the non-DAOSD group after comparing baseline values with values after 16 weeks of dupilumab treatment (0.16×109/l (IQR –0.05–0.53) and 0.04×109/l (IQR –0.08–0.26), respectively, p = 0.001, Table II).
DISCUSSION
In this large prospective cohort study, 32.4% of patients with AD developed DAOSD. We found that a history of any eye disease, other than a history of self-reported episodic acute allergic conjunctivitis, combined with the use of ophthalmic medication indicating current disease at baseline, was associated with the development of DAOSD. In addition, the development of DAOSD was associated with higher clinical effectiveness of dupilumab at 4 and 16 weeks, and a stronger increase in blood eosinophils at 16 weeks of treatment compared with the non-DAOSD group.
In both clinical trial populations and data from small daily practice studies, a prior history of allergic conjunctivitis is a frequently reported risk factor for the development of DAOSD (9, 12–14). However, in most studies, the definition of allergic conjunctivitis is very broad. The current study used a more refined classification of allergic conjunctivitis and separated a history of self-reported episodic acute allergic conjunctivitis from a history of more chronic allergic eye disease, such as atopic keratoconjunctivitis and vernal keratoconjunctivitis (mostly confirmed by an ophthalmologist). In the current study, only univariate analysis showed a statistically significant association between the development of DAOSD and a history of self-reported episodic acute allergic conjunctivitis. This association was not significant in the multivariate analysis, possibly due to correction for confounding in the multivariate analysis, like other atopic diseases or more severe ophthalmological pathology. Multivariate analyses showed a significant association between the development of DAOSD and a history of any eye disease (history of self-reported episodic acute allergic conjunctivitis excluded) combined with use of ophthalmic drugs at baseline. Ongoing ophthalmic treatment for previous eye disease might indicate ongoing ophthalmic pathology, leading to a higher risk of development of DAOSD. It is not known why patients with allergic and non-allergic eye disease combined with ophthalmic drugs are at risk of DAOSD. However, it is known that preservatives in eye drops cause conjunctival allergy and irritation (22). Whether this hypersensitivity is related to DAOSD development is unclear, since no information is available about the presence of preservatives in eye drops in the current study series. In addition, some of the eye diseases classified as non-allergic might indirectly be related to AD or its treatment, such as cataract and glaucoma (23, 24)
Another frequently reported associated risk factor with the development of DAOSD in patients with AD is AD severity at baseline. Akinlade et al. (9) performed a post hoc analysis of published clinical trial data on dupilumab treatment in AD regarding the development of DAOSD. The conclusion of this post hoc analysis was that a higher incidence of DAOSD in patients with AD was associated with higher baseline AD severity. However, Akinlade et al. (9) used different and inconsistent banding of the EASI scores, making the comparison of these results with the current study difficult. In the current study, EASI scores were classified according to the validated HOME criteria (16), and no relationship was found between the baseline severity of AD and the development of DAOSD. In addition, there is an important difference in patient population between the studies; Akinlade et al. (9) included participants of clinical trials, while the current study included patients in daily practice, representing a more diverse population regarding baseline AD severity.
Ariens et al. (5) also found that development of DAOSD was significantly associated with higher EASI scores at baseline. Despite the fact that these patients also participated in the BioDay registry, the results are not comparable with our current study. The selected cohort of Ariens et al. (5) is smaller and the patients were participating in an Early Access programme, which allowed the patients with most severe AD to start dupilumab ahead of market access. Therefore, these patients had high EASI scores at baseline, comparable with the clinical trial population, while the current cohort was more diverse.
Although baseline AD severity was not significantly associated with the development of DAOSD in the current study, patients showing a larger decrease in EASI score at 4 weeks and 16 weeks of treatment more often developed DAOSD. This might indicate that higher clinical effectiveness of dupilumab in AD is associated with higher risk of developing DAOSD. We previously investigated the long-term follow-up of DAOSD and found improvement in DAOSD after prolongation of the dosing interval of dupilumab to 300 mg every three to 5 weeks (15). Since dose reduction improved signs and symptoms of the DAOSD, and EASI reduced significantly more in the group of dupilumab treated patients that developed DAOSD, it might be possible that these patients have higher serum levels of dupilumab, and are thus relatively overdosed.
Another reported potential risk factor to develop DAOSD is the presence of eyelid eczema. Touhouche et al. (14) reported that this was significantly associated with the occurrence of DAOSD, with an OR of 8.7 (95% CI 1.8–40.6). This is in line with findings of the current univariate analysis, in which eyelid involvement in AD in the past year was statistically more present in the group of patients who developed DAOSD (OR 3.73; 95% CI 1.70–8.19, p = 0.004). Dogru et al. (25) investigated ocular surface disorders in patients with severe AD. They described the presence of eyelid eczema, which was seen in 476/724 eyes (65.7%), as one of the most dominant ocular disease in patients with severe AD. Another study of Dogru et al. (26) investigated conjunctival impression cytology samples in patients with AD. They concluded that patients with AD with facial eczema showed significantly higher grades of conjunctival squamous metaplasia.
In the current cohort, significantly more patients who developed DAOSD had eyelid eczema in the past year. This might indicate that these patients had (undiagnosed) ocular surface disease, which exacerbated during the use of dupilumab. For this reason, it is important to ask patients about presence of redness, tearing, itch, burning sense, photophobia, and painful eyes before the start of dupilumab. Low-threshold referral to the ophthalmologist is recommended, especially in patients with a history of any eye disease that are currently using ophthalmic treatment and patients having eyelid eczema in the past year.
To the best of our knowledge, this is the first large prospective daily practice cohort study that investigated risk factors associated with the development of DAOSD in patients with AD. Patients were seen by physicians aware of the ophthalmological comorbidity in AD, and standardized procedures were followed, leading to accurate data collection. We included many different variables, and the current prospective database provided information about ophthalmic treatment at the start of dupilumab. The current study has some limitations. Firstly, no ophthalmological slit lamp examination was performed before the start of dupilumab. Therefore, only patient-reported information regarding the ophthalmological history was available, and pre-existing ophthalmological pathology, which can only be found during ophthalmic slit-lamp examination, could not be excluded. Secondly, the variable AD eyelid involvement in the past year had many missing values and was therefore not included in the multivariate logistic analyses. Sensitivity analyses evaluated the effect of the inclusion of eyelid involvement in the past year. However, this showed minor differences (see Table SII). Thirdly, only the absence or presence of DAOSD was reported during an outpatient visit. As a result, the exact date of onset of DAOSD is missing. However, previous literature reported that most patients developed DAOSD within the first 16 weeks of treatment (7, 9, 15). All of our included patients had a follow-up duration of at least 16 weeks, and the absence or presence of DAOSD was reported, making the information about the date of onset of DAOSD less relevant for the primary analyses. Fourthly, both a history of any eye disease and ocular medication are broad definitions. However, the subgroups of individual eye diseases and individual ocular medications were too small to analyse. Therefore, these subgroups were not included in the analysis. Lastly, for some variables, like a history of rosacea, no conclusions can be drawn due to the small number of patients. Larger cohorts or other comparable cohort studies are necessary to support the current results.
In conclusion, this large prospective cohort study in which one-third of the patients with AD developed DAOSD, showed that a history of any eye disease, but not a history of self-reported episodic acute allergic conjunctivitis, combined with the use of ophthalmic medication at baseline, was associated with the development of DAOSD. In addition, high effectiveness of dupilumab appeared to be associated with development of DAOSD. Future studies are warranted to investigate the association between specific ophthalmological characteristics at baseline and the development of DAOSD and to determine (preventive) treatment strategies.
ACKNOWLEDGEMENTS
The authors thank Andrew Walker for critically reading the manuscript. Patients included in this manuscript participated in the BioDay registry sponsored by Sanofi Genzyme.
Conflicts of interest: REA has nothing to disclose. CMvL is a speaker for Sanofi Genzyme. LPvdR has nothing to disclose. DSB is a speaker for Sanofi Genzyme and LEO Pharma. LSS has nothing to disclose. NPAZ has nothing to disclose. MLAS is an advisory board member and/or speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme. GLER has nothing to disclose. Angelique N. Voorberg has nothing to disclose. MK is an advisory board member of LEO Pharma. IMH is an advisory board member, and/or speaker for AbbVie, Eli Lilly, Leo Pharma and Sanofi-Genzyme. MdG is a principal investigator and advisory board member and/or speaker for Sanofi Genzyme and Regeneron Pharmaceuticals and LEO Pharma, and is an advisory board member for Eli Lilly. JLT is a speaker for Sanofi Genzyme and LEO Pharma. JHdB received research funding from Abbvie; this is outside the submitted work. MSdB-W is a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Arena, Eli Lilly, Galderma, Janssen, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme.
REFERENCES
- de Bruin-Weller M, Thaci D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178: 1083–1101.
- Faiz S, Giovannelli J, Podevin C, Jachiet M, Bouaziz JD, Reguiai Z, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol 2019; 81: 143–151.
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287–2303.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348.
- Ariens LF, van der Schaft J, Spekhorst LS, Bakker DS, Romeijn GLE, Kouwenhoven TA, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-weeks results from the Dutch BioDay registry. J Am Acad Dermatol 2021; 84: 1000–1009.
- Olesen CM, Holm JG, Norreslet LB, Serup JV, Thomsen SF, Agner T. Treatment of atopic dermatitis with dupilumab: experience from a tertiary referral centre. J Eur Acad Dermatol Venereol 2019; 33: 1562–1568.
- Popiela MZ, Barbara R, Turnbull AMJ, Corden E, Martinez-Falero BS, O’Driscoll D, et al. Dupilumab-associated ocular surface disease: presentation, management and long-term sequelae. Eye (Lond) 2021; 35: 3277–3284.
- Bohner A, Topham C, Strunck J, Haynes D, Brazil M, Clements J, et al. Dupilumab-associated ocular surface disease: clinical characteristics, treatment, and follow-up. Cornea 2021; 40: 584–589.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019; 181: 459–473.
- Ariens LFM, van der Schaft J, Bakker DS, Balak D, Romeijn MLE, Kouwenhoven T, et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy 2020; 75: 116–126.
- Treister AD, Kraff-Cooper C, Lio PA. Risk factors for dupilumab-associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol 2018; 154: 1208–1211.
- Uchida H, Kamata M, Nagata M, Fukaya S, Hayashi K, Fukuyasu A, et al. Conjunctivitis in patients with atopic dermatitis treated with dupilumab is associated with higher baseline serum levels of immunoglobulin E and thymus and activation-regulated chemokine but not clinical severity in a real-world setting. J Am Acad Dermatol 2020; 82: 1247–1249.
- Nettis E, Bonzano L, Patella V, Detoraki A, Trerotoli P, Lombardo C, et al. Dupilumab-associated conjunctivitis in patients with atopic dermatitis: a multicenter real-life experience. J Investig Allergol Clin Immunol 2020; 30: 201–204.
- Touhouche AT, Cassagne M, Berard E, Giordano-Labadie F, Didier A, Fournie P, et al. Incidence and risk factors for dupilumab associated ocular adverse events: a real-life prospective study. J Eur Acad Dermatol Venereol 2021; 35: 172–179.
- Achten R, Bakker D, Ariens L, Lans A, Thijs J, van der Schaft J, et al. Long-term follow-up and treatment outcomes of conjunctivitis during dupilumab treatment in patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract 2021; 9: 1389–1392.e2.
- Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017; 177: 1316–1321.
- Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin-17 levels? Br J Dermatol 2018; 178: 1220.
- Thyssen JP, Toft PB, Halling-Overgaard AS, Gislason GH, Skov L, Egeberg A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol 2017; 77: 280–286.e1.
- Simonetti O, Radi G, Diotallevi F, Molinelli E, Rizzetto G, Offidani A. Prevention of conjunctivitis in patients with atopic dermatitis undergoing treatment with dupilumab: an Italian single centre experience. Clin Exp Dermatol 2021; 46: 939–940.
- Harrell Jr FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis: Springer; 2015.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Strat Soc 1995; 57: 289–300.
- Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol 2009; 9: 447–453.
- Beck KM, Seitzman GD, Yang EJ, Sanchez IM, Liao W. Ocular co-morbidities of atopic dermatitis. Part I: associated ocular diseases. Am J Clin Dermatol 2019; 20: 797–805.
- Beck KM, Seitzman GD, Yang EJ, Sanchez IM, Liao W. Ocular co-morbidities of atopic dermatitis. Part II: ocular disease secondary to treatments. Am J Clin Dermatol 2019; 20: 807–815.
- Dogru M, Nakagawa N, Tetsumoto K, Katakami C, Yamamoto M. Ocular surface disease in atopic dermatitis. Jpn J Ophthalmol 1999; 43: 53–57.
- Dogru M, Katakami C, Nakagawa N, Tetsumoto K, Yamamoto M. Impression cytology in atopic dermatitis. Ophthalmology 1998; 105: 1478–1484.