ORIGINAL REPORT
Relationship Between Standardized Measures of Chronic Kidney Disease-associated Pruritus Intensity and Health-related Quality of Life Measured with the EQ-5D Questionnaire: A Mapping Study
Monica HERNANDEZ ALAVA1, Alessandro SASSO1,2, Pann Ei HNYNN SI1,3, Matthew GITTUS1,3, Richard POWELL4, Louese DUNN3, Praveen THOKALA1 and James FOTHERINGHAM1,3
1School of Health and Related Research, University of Sheffield, Sheffield, UK, 2European Commission, Joint Research Center (JRC), Ispra, Italy, 3Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Trust, Sheffield, UK and 4University Hospitals Plymouth NHS Trust, Plymouth, UK
Chronic kidney disease-associated pruritus is linked with decreased health-related quality of life assessed using disease-specific instruments. The extent to which worsening pruritus reduces generic quality of life assessed using the EQ-5D instrument is unknown. Prevalent kidney failure patients receiving in-centre haemodialysis from 5 centres completed the EQ-5D-5L quality of life measure, worst Itching Intensity Numerical Rating Scale and 5-D itch pruritus instruments. Latent class models were used to identify clusters of patients with similarly affected body parts, and mixture models were used to map the pruritus measures to the EQ-5D. Data on 487 respondents were obtained. Latent class analysis identified 3 groups of patients who had progressively worsening severity and an increasing number of body parts affected. Although the worst itching intensity numerical rating scale and 5-D itch instruments correlated with each other, only the latter had a strong relationship with EQ-5D. When controlling for age, sex, diabetes and years receiving dialysis, the meanpredicted EQ-5D utility (1: perfect health, 0: dead) decreased progressively from 0.69 to 0.41. These findings suggest that pruritus instruments that include domains capturing how the individual is physically, mentally and socially affected by their pruritus, in addition to severity, more closely approximate the EQ-5D generic quality of life measure.
Key words: chronic kidney disease; health-related quality of life; pruritus.
SIGNIFICANCE
More than one-third of people with kidney failure who receive dialysis experience itching (pruritus). Itching is commonly measured using scores: some measures only assess intensity, while others ask the person how itching affects other areas of their life. These areas are quite specific to itching, unlike the data captured by the more general EQ-5D, which is commonly used to make reimbursement decisions about new treatments. This study shows that as measures that ask how itching affects life more broadly, such as the 5-D itch, get worse, so does EQ-5D. Measures that only assess intensity should not be used as the sole measure of itch in studies of new treatments.
Citation: Acta Derm Venereol 2023; 103: adv11604. DOI https://doi.org/10.2340/actadv.v103.11604.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 29, 2023; Published: Sep 20, 2023
Corr: James Fotheringham, School of Health and Related Research, University of Sheffield, Regent Court, Regent Street, Sheffield S1 4DA, UK. E-mail: j.fotheringham@sheffield.ac.uk
Competing interests and funding: JF has received honoraria from Fresenius, Novartis and Vifor Pharma and conducts research funded by Baxter and Boehringer Ingelheim.
INTRODUCTION
More than one-third of people with kidney failure receiving haemodialysis (HD) are at least moderately bothered by uraemic or chronic kidney disease-associated pruritus (CKD-aP) (1, 2). There is increasing recognition that urea, other uraemic toxins and phosphate do not have a mechanistic role in the development of pruritus in this patient group, leading to the revised term CKD-aP. Existing evidence suggests that the severity of the pruritus is associated with depression, poor sleep quality, increased mortality and reduced health-related quality of life (HRQoL) (3–5). Patients and clinicians have repeatedly prioritized the treatment of CKD-aP (6). Clinical management of this condition is delivered by both nephrologists and dermatologists, and includes dialysis optimization, emollients, topical agents, antihistamines, gabapentinoids and the only licensed treatment for CKD-aP, difelikefalin (7–9).
CKD-aP can be assessed with a range of patient-reported measures (PROMs), with the most commonly used being the severity-based verbal rating scale and Worst Itching Intensity Numerical Rating Scale (WI-NRS), and disease-specific quality of life measures, such as the 5-D itch and SKINDEX-10 (10–12). Although these capture domains including severity and how pruritus affects body parts, mental health and social functioning, they are not preferred measures when evaluating the cost-effectiveness of therapies. Reimbursement agencies and other decision-makers prefer generic instruments, such as EQ-5D, to perform cost-utility analyses (13). In practice, this requires EQ-5D to also be collected, or in its absence other dermatological measures to be mapped to EQ-5D using an appropriate statistical method (14).
Many health economic models that have led to successful policy decisions on dermatological treatments have adopted a severity-focused structure, and been informed by generic measures of HRQoL (15, 16). The extent to which disease-specific measures capturing the broader impact of dermatological conditions on the patient relate to generic measures used for cost-effectiveness analyses is unknown. To explore these issues and inform reimbursement decisions, we report here a UK multi-centre primary data collection of these instruments in a cohort study of prevalent people with kidney failure receiving haemodialysis, mapping the WI-NRS and 5-D itch to EQ-5D and how the domains including body parts affected are associated with generic HRQoL. Understanding these relationships could inform the design, conduct and analyses of interventional and observational studies of HRQoL in people with dermatological conditions, particularly when trying to determine the cost-effectiveness of treatments (17).
MATERIALS AND METHODS
Study design, location and participants
This cross-sectional study collected established patient-reported outcome measures for pruritus across 5 UK centres between November 2020 and July 2021. The primary aim of the study was to generate the first published mapping (a conversion algorithm) between measures of CKD-aP and EQ-5D HRQoL. Inclusion criteria included individuals established on in-centre haemodialysis for kidney failure for more than 3 months, and over the age of 18 years. Exclusion criteria included individuals who were unable to give consent or understand written English. Having pruritus now or in the past was not an inclusion criterion, as the benefit of having no CKD-aP also needed to be quantified. Those who agreed to participate were consented to the study by trained Good Clinical Practice-certified members of the research team and then given questionnaires to complete either during initial encounter or to take home for completion in their own time. Because two-thirds of people receiving dialysis do so via a fistula formed in their arm, the research team assisted participants who chose to complete the questionnaires while receiving haemodialysis, but were not able to complete the written instruments themselves.
Data collection and instruments
The study collected 4 pruritus-related measures and EQ-5D-5L data. The Worst Itching Intensity Numerical Rating Scale (WI-NRS) asks the respondent to indicate the highest intensity of their pruritus over the last 24 h, from 0 (no itching) to 10 (worst itching imaginable), and is a recommended instrument for the evaluation of pruritus in clinical trials (10, 18). The 5-D itch scale is a 5-domain disease-specific instrument evaluating the duration, degree (severity), direction (improving, unaltered or worsening), disability (impact on sleep, leisure/social, housework/errands and school/work activities) and distribution (body part affected), resulting in a score between 5 (least) and 25 (most impacted) (11). The degree domain is a 5-level verbal rating scale that also features in other broader measures of kidney disease-related quality of life, which was used to stratify patients for descriptive statistics. The 5-D itch has a 2-week recall period. Data on 2 additional patient-reported pruritus measures, a verbal rating scale and the SKINDEX-10, were collected (12, 19). These 2 measures are not included in the mapping study as they have been shown to not add additional information, but we exploit their informational content for data checking and cleaning (20). The EQ-5D-5L is a 5-item standardized instrument developed as a measure of generic health-related quality of life at the time of completion and it consists of a descriptive system that comprises 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, for which respondents select 1 of 5 levels, from no problems to unbearable or being unable to perform the domain (13). A country-specific utility value based on general public preferences regarding quality and length of life can be attached to the responses (21). The value of zero represents health states equivalent to being dead and the utility values range from negative (for health states considered worse than being dead) to 1 (full health). This generic measure and associated utility values are used to compare health-related quality of life between groups of individuals with chronic conditions, particularly for health economic evaluations. The data collection instrument is provided in Appendix S1. In addition, the researchers also collected data on patient demographics and comorbidities, dialysis prescription and CKD-aP-associated medication use. The presence of other skin conditions was not collected.
The target number of patients included in the data collection was informed by considerations of the mapping models requiring estimation. An existing dataset from the SHAREHD randomized controlled trial (22) was accessed, which included EQ5D-5L, and pruritus was measured using an ordinal scale from none to overwhelming. These data were used to infer the number of patients needed to ensure coverage of the full range of severity and to successfully estimate mapping models based on mixture distributions (23).
Statistical analysis
Because of concerns regarding the value set for the EQ-5D-5L, data on EQ-5D-5L were converted into an EQ-5D-3L UK utility using the van Hout crosswalk, adhering to The National Institute for Health and Care Excellence (NICE) guidance at the time (24, 25). More recent NICE guidance, published in 2022, suggests a different mapping model for conversion (26). However, it has been shown that the differences between the mapping models are small and should not affect the general conclusions of the presented analyses (27). To identify individuals with specific combinations of body parts affected by CKD-aP that may have different health-related quality of life values, a latent class model was used with the 16 body parts screened for in the 5-D itch. Using the combination of Bayesian Information Criterion (BIC), entropy and log likelihood tests comparing models with different number of classes, a model distributing respondents across 3 classes was selected (models with up to 5 classes were estimated).
To estimate the relationship between the CKD-aP measures (WI-NRS and 5-D itch) and the EQ-5D, the Adjusted Limited Dependent Variable Mixture Model (ALDVMM) was used. Preference-based measures, such as EQ-5D, have challenging distributional characteristics for statistical analysis (27, 28). Utility data is skewed, bounded at 1 (full health) and at the bottom by the value of the worst health state described by the EQ-5D instrument and often presents a large number of observations at perfect health, followed immediately by a gap to the next utility value. The ALDVMM is a flexible semiparametric model developed specifically to deal with these key characteristics of the EQ-5D distribution and has been shown to outperform other models when mapping to a number of generic preference-based measures (27, 28). The model comprises separate distributions (or components) with different parameters mixed according to some weighting probabilities. The covariates can affect the mean of each component distribution, the probability of component membership or both. Finding the optimal model involves estimating ALDVMMs for different numbers of component distributions using maximum likelihood and then selecting the optimal number of components according to a combination of information criteria, such as Akaike Information Criterion (AIC) and BIC; measures of model fit, such as mean absolute error (MAE) and root mean squared error (RMSE); and graphical methods to select the optimal mapping models (27). In selecting the most appropriate model, it is important to use a range of measures and take into account how the mapping model will be used. Between 2 and 4 components and similar specifications were used for both measures of pruritus. For each model several estimation rounds were run, each using from 200 to 2,500 starting points, until no further improvement in the likelihood function could be found. In order to maximize the applicability of the mapping to other populations, adjustment variables (age, sex, time on dialysis and diabetes status) were incorporated a priori in the mapping models. In addition, exploratory analyses were performed to identify individuals who had never had CKD-aP, as the mapping was most likely to estimate HRQoL benefits for those whose CKD-aP had improved. All the statistical analyses are performed in Stata 17.0 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.). Mapping models are estimated using the community-contributed Stata command ALDVMM (29).
RESULTS
Across the 5 participating centres that collectively care for 2,326 people on haemodialysis, 523 were approached to participate in the study, of whom 487 consented.
Respondent, pruritus and health-related quality of life characteristics
EQ-5D-5L, WI-NRS and 5-D itch had data missing for 9, 1 and 24 patients, respectively. Amongst the 24 patients with a missing 5-D itch score, the majority (17) reported no itching in the last week (verbal rating scale) or in the last 24 h (WI-NRS).
Fig. 1 plots the distribution of EQ-5D-3L and includes the typical characteristics of a large peak at full health (12% of the sample), a gap and a skewed, multimodal distribution. The mean utility of 0.590 is low relative to full health, and approximately 5% of patients report health states with associated negative values (worse than death).
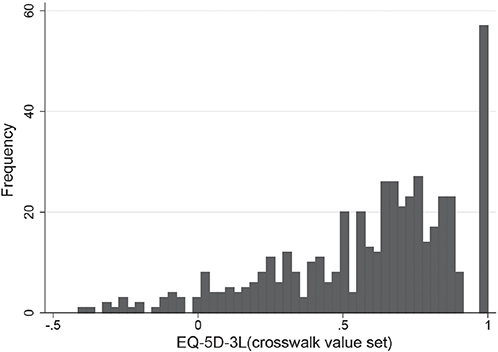
Fig. 1. EQ-5D-3L sample distribution using the van Hout crosswalk value set.
Fig. 2 shows the distributions of WI-NRS and 5-D itch, respectively. For both measures, higher scores represent a greater impact of pruritus on the patient, and so they are expected to move in the opposite direction to EQ-5D. Using the WI-NRS, a large proportion of patients (38%) reported no itching in the last 24 h with the remaining patients fairly evenly spread across the remaining 10 groups of pruritus intensity, as measured by WI-NRS. Respondents with a 5-D itch score of 5 represent people reporting absence of pruritus in the previous 2 weeks and who considered their itching completely resolved relative to the previous month. The largest peak in the 5-D itch index appears at a value of 8, completed by people who appear to have no recent experience of pruritus. A relatively large group of respondents (n=65) who tick “Not present” in the degree dimension of 5-D itch and have no evidence of pruritus in any of the other collected variables in the survey, respond “4” “unchanged” in the direction dimension rather than the low scoring but inappropriate “completely resolved”, resulting in a 5-D itch score of 8. As patients with no previous experience of pruritus might not accurately reflect the populations in which the mapping will be used, they were excluded from further analyses, resulting in a final sample size of 377 observations.
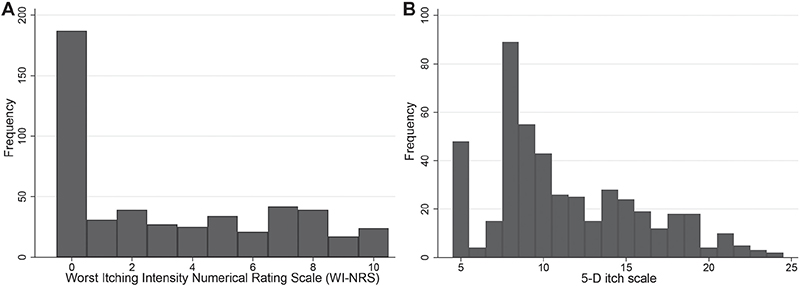
Fig. 2. Sample distributions of (A) the Worst Itching Intensity Numerical Rating Scale (WI-NRS) and (B) the 5-D itch scale.
Table I presents summary statistics for different groups of CKD-aP severity, defined according to the verbal rating scale included in the 5-D itch questionnaire (degree of itching intensity) and notes a higher proportion of females and people with diabetes as pruritus severity worsens. The mean EQ-5D-3L decreases as the pruritus severity increases within the sample of individuals who have pruritus, but it is slightly lower for people with no pruritus compared with people with mild pruritus.
As expected, mean EQ-5D-3L tends to decline as the 5-D itch index score increases (Fig. 3), and there is a strong relationship between the WI-NRS and 5-D itch (Fig. 4). The panel on the right depicts the relationship of EQ-5D-3L with WI-NRS. Unlike the 5-D itch index, mean EQ-5D-3L fluctuates as WI-NRS increases and does not show a clear downward trend (Fig. 4).
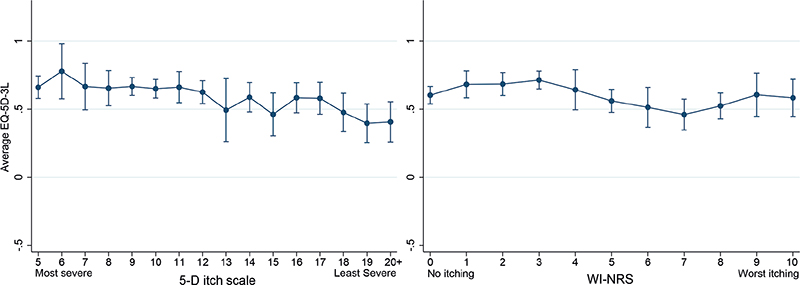
Fig. 3. Estimated means of EQ-5D-3L and 95% bootstrapped confidence intervals by 5-D itch index (left) and Worst Itching Intensity Numerical Rating Scale (WI-NRS) (right) (5-D itch scale 20 includes all observations in the range 20–25).
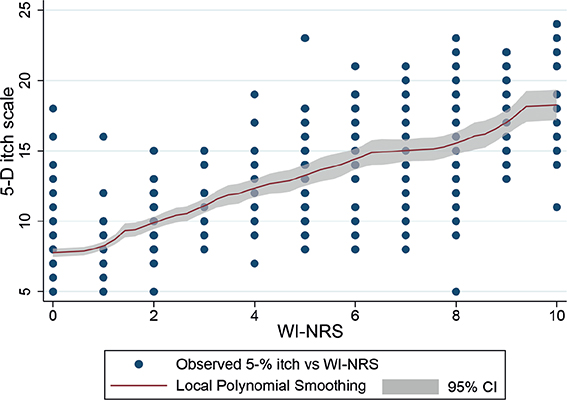
Fig. 4. Scatterplot of 5-D itch against Worst Itching Intensity Numerical Rating Scale (WI-NRS) and local polynomial smoothing with 95% confidence intervals (95% CI).
Analysis of the 3 groups of respondents identified with latent class analysis using body parts affected (Fig. 5) revealed 3 groups with increasing severity of pruritus. The overall population had high probabilities of head, back, lower limbs and upper limbs being affected. As severity worsened (class 2 and class 3) the probability of other body parts being affected increased. Within each of the latent profiles, the probability of itching affecting the back is consistently the highest. Meanwhile WI-NRS, 5-D itch and EQ-5D all worsened when moving from class 1 to class 3, as shown in Table II.
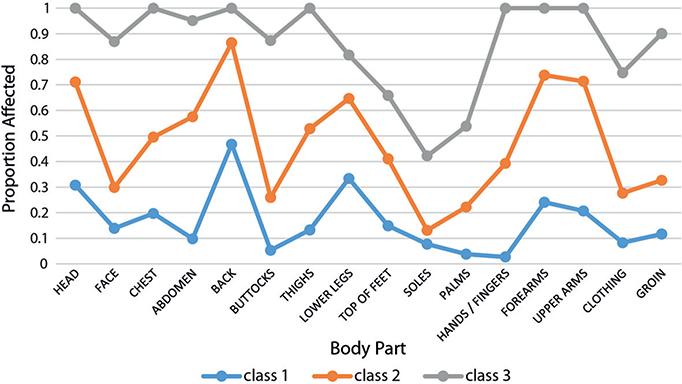
Fig. 5. Distribution of the 16 body parts assessed in the 5-D itch according to 3 populations identified using latent class analysis.
Mapping models
Two alternative EQ-5D-3L models were developed, mapping from: (i) the 5-D itch score; and (ii) the WI-NRS. Full details of the mapping models including variable coefficients and measures of model fit are shown in Tables SI–III. A 3- and a 2-component model are selected for the mappings, including 5-D itch score and WI-NRS, respectively, in addition to age, sex, the presence of diabetes and the number of years on dialysis. Fig. 6 presents plots of the models’ predictions by each pruritus measure. The 5-D itch mapping model (Fig. 6a) is able to reproduce the mean utilities in the data quite closely. However, Fig. 6b shows clear systematic problems in the predictions by WI-NRS groups, reflected in inferior measures of model fit and the general lack of statistical significance of the coefficients for the WI-NRS in the mapping model, suggesting that WI-NRS is not a good predictor of EQ-5D-3L in these data. A mapping including both measures of pruritus was also developed; however, this did not significantly improve the performance of the mapping and so is not reported here.
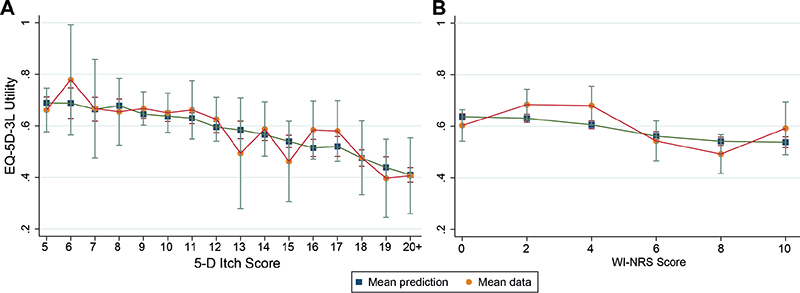
Fig. 6. Comparison of EQ-5D-3L observed means by (A) 5-D itch score and (B) Worst Itchiness Numerical Rating Scale (WI-NRS) [orange circles], and the EQ-5D-3L predicted means from the models [blue squares].
DISCUSSION
Patients, clinicians and existing literature highlight the relationship between HRQoL and CKD-aP, arguing for further research and the development and utilization of targeted therapies; however, it has not been possible to relate measures of CKD-aP severity and the EQ-5D. Using data from 377 patients, the current study illustrated that CKD-aP measures are associated with a generic measure of HRQoL. CKD-aP measures, which include how pruritus impacts other aspects of life (e.g. the 5-D itch disability domain) predict EQ-5D better, while measures of pruritus severity in isolation (e.g. WI-NRS) do not predict EQ-5D well and may lead to an underestimation of the impact of pruritus on the patient. Our latent class analysis suggests that, as CKD-aP severity worsens, the number of body parts affected increases, but the distribution of body parts affected seems to be relatively constant.
Dermatological stakeholders largely recommend severity-focused pruritus instruments for clinical trials, such as the WI-NRS, which we demonstrate cannot predict EQ-5D HRQoL that is relied upon by reimbursement agencies and other decision-makers (18). WI-NRS is a single-item measurement of the intensity of the worst itching in the past 24 h and might not capture additional health-related issues, such as the impact of itch on sleep quality that multidimensional measures, such as 5-D itch, capture (27). However, the relative ease with which the WI-NRS can be completed and high completion rate by the respondent cannot be ignored (30). The number and nature of the questions in the 5-D itch make it more challenging, and this, combined with structural issues that manifest when individuals who have no recent pruritus complete it, may lead to missing or misleading values.
Systematic reviews and these trials have shown that disease-specific HRQoL measures are sensitive to changes in pruritus severity in CKD-aP, but the degree to which EQ-5D improves with lessening CKD-aP severity within individuals remains unobserved (5): open-label trial data for the use of difelikefalin for CKD-aP that also included EQ-5D-5L is available for comparison (31): participants being treated with difelikefalin had a clinically meaningful change in EQ-5D utility of 0.04, and 73.7% of patients on difelikefalin improved their WI-NRS by ≥3 points (comparable to 1 Verbal Rating Scale level). Due to the nature of the trial’s reported data and the cross-sectional study design twe are unable to confirm if changes in CKD-aP severity in response to therapies yield similar improvements in HRQoL as those estimated from our analyses. Questions in the 5D-itch pertaining to the evolution of pruritus severity, and greater conceptual overlap in domains with the EQ-5D than the WI-NRS, have resulted in better performance of the 5D-itch in estimating generic HRQoL. Our population EQ-5D utility value (0.590) is comparable to randomised controlled trials, systematic reviews and data collected in other UK kidney failure populations (22, 32).
The strengths of this study are that it explores highly prevalent instruments for the assessment of CKD-aP and uses methods widely regarded as appropriate to relate the measures reported. Additional strengths include that primary data collection was for the purpose of this analysis and there were broad inclusion criteria for participants which maximize its real-world applicability. Weaknesses include that we did not capture the presence of primary skin conditions that may cause pruritus, but based on the incidence and prevalence of psoriasis and eczema, respectively, it would be reasonable to assume that less than 5% of respondents had these conditions (33, 34). Although the merging of severe and overwhelming verbal rating scale groups for reporting purposes was necessitated by the number of observations in the data, the resulting 4 levels are those originally described in the derivation paper for this instrument. Our analyses and their conclusions are limited to people affected by pruritus caused by CKD-aP, and other conditions or patient groups may behave differently. As there is some variation in the prevalence of CKD-aP (2) and HRQoL (35) globally, the size of the relationship between the two, estimated in this study, could be considered relevant to the measures studied and the UK only. However, a systematic review on the relationship between CKD-aP and HRQoL identified positive studies from multiple countries (5). The current recommended mapping for use in NICE health technology evaluations is different to the one used here, but the differences are small and are not expected to affect the general conclusions of this paper (26).
Considering the policy and practice implications of these findings, those wishing to assess the disease-specific impact of a condition on disease-specific HRQoL should use a disease-specific instrument that also provides a more reliable means of mapping to HRQoL than severity-based measures alone. Regulators have already accepted the pruritus measures we report herein (36). Some reimbursement agencies allow the use of non-generic HRQoL instruments if there is sufficient justification and the extent to which this criteria is met remains unassessed (26), and the perceived insensitivity of these measures to interventions in people on haemodialysis and prioritization by this population of other patient-reported outcome measures should be acknowledged (37, 38). These data justify recommendations made by others that, when an individual with CKD-aP is identified, the impact on HRQoL should be assessed before initiating therapy, but the distribution of body parts affected is unlikely to be relevant (36). Future research should consider whether the relative contribution of the affected body parts captured by the 5-D itch should be revisited to maximize its relationship with HRQoL, address the structural problem with the 5-D itch to make it more translatable to pruritus populations without pruritus, and consider the influence of the recall period across these measures. The content validity and recall period of the WI-NRS and 5-D itch instruments should be explored considering the disagreement observed in some patients (Fig. 4). Future data collection in similar settings could include a question routing patients to the 5-D itch questionnaire after confirmation of past experience of pruritus.
ACKNOWLEDGEMENTS
This research was funded by an unconditional research grant from Vifor Pharma, Switzerland.
IRB approval status: Ethical approval was granted by North West – Greater Manchester, ethics committee (Integrated Research Application System Reference: 285714) and written consent was obtained from the participants.
REFERENCES
- Swarna SS, Aziz K, Zubair T, Qadir N, Khan M. Pruritus associated with chronic kidney disease: a comprehensive literature review. Cureus 2019; 11: e5256.
- Sukul N, Karaboyas A, Csomor PA, Schaufler T, Wen W, Menzaghi F, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2021; 3: 42–53.e41.
- Rayner HC, Larkina M, Wang M, Graham-Brown M, van der Veer SN, Ecder T, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 2000–2007.
- Pisoni RL, Wikström B, Elder SJ, Akizawa T, Asano Y, Keen ML, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21: 3495–3505.
- Poku E, Harnan S, Rooney G, James MM, Hernández-Alava M, Schaufler T, et al. The relationship between chronic kidney disease-associated pruritus and health-related quality of life: a systematic review. Clin Kidney J 2022; 15: 484–499.
- Manns B, Hemmelgarn B, Lillie E, Dip SC, Cyr A, Gladish M, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014; 9: 1813–1821.
- Lipman ZM, Paramasivam V, Yosipovitch G, Germain MJ. Clinical management of chronic kidney disease-associated pruritus: current treatment options and future approaches. Clin Kidney J 2021; 14: i16–i22.
- Millington GWM, Collins A, Lovell CR, Leslie TA, Yong ASW, Morgan JD, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol 2018; 178: 34–60.
- Ständer S, Zeidler C, Augustin M, Darsow U, Kremer AE, Legat SK, et al. Diagnostik und Therapie des chronischen Pruritus. S2k-LL AWMF-Register 2017; 13: 048.
- Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol 2015; 54: 715–722.
- Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol 2010; 162: 587–593.
- Mathur VS, Lindberg J, Germain M, Block G, Tumlin J, Smith M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1410–1419.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736.
- Hernández Alava M, Wailoo A, Wolfe F, Michaud K. A comparison of direct and indirect methods for the estimation of health utilities from clinical outcomes. Med Decis Making 2014; 34: 919–930.
- Heinz KC, Willems D, Hiligsmann M. Economic evaluation of a JAK inhibitor compared to a monoclonal antibody for treatment of moderate-to-severe atopic dermatitis from a UK perspective. J Med Econ 2022; 25: 491–502.
- Thokala P, Hnynn Si PE, Hernandez Alava M, Sasso A, Schaufler T, Soro M, et al. Cost effectiveness of difelikefalin compared to standard care for treating chronic kidney disease associated pruritus (CKD-aP) in people with kidney failure receiving haemodialysis. Pharmacoeconomics 2023; 41: 457–466.
- Whang KA, Khanna R, Williams KA, Mahadevan V, Semenov Y, Kwatra SG. Health-related QOL and economic burden of chronic pruritus. J Invest Dermatol 2021; 141: 754–760.e1.
- Ständer S, Augustin M, Reich A, Blome C, Ebata T, Phan NQ, et al. Pruritus assessment in clinical trials: consensus recommendations from the International Forum for the Study of Itch (IFSI) Special Interest Group Scoring Itch in Clinical Trials. Acta Derm Venereol 2013; 93: 509–514.
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92: 497–501.
- Lopes MB, Karaboyas A, Sukul N, Tsuruya K, Al Salmi I, Asgari E, et al. Utility of a single itch-related question and the Skindex-10 questionnaire for assessing pruritus and predicting health-related quality of life in patients receiving hemodialysis. Kidney Med 2022; 4: 100476.
- Hernandez Alava M, Pudney S, Wailoo A. The EQ-5D-5L value set for England: findings of a quality assurance program. Value Health 2020; 23: 642–648.
- Fotheringham J, Barnes T, Dunn L, Lee S, Ariss S, Young T, et al. A breakthrough series collaborative to increase patient participation with hemodialysis tasks: a stepped wedge cluster randomised controlled trial. PLoS One 2021; 16: e0253966.
- Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, et al. Mapping to estimate health-state utility from non-preference-based outcome measures: an ISPOR good practices for outcomes research task force report. Value Health 2017; 20: 18–27.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012; 15: 708–715.
- National Institute for H, Care E. NICE process and methods guides. Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence (NICE); 2013.
- Dawoud D, Lamb A, Moore A, Bregman C, Rupniewska E, Paling T, et al. Capturing what matters: updating NICE methods guidance on measuring and valuing health. Qual Life Res 2022; 31: 2167–2173.
- Hernández Alava M, Wailoo A, Pudney S, Gray L, Manca A. Mapping clinical outcomes to generic preference-based outcome measures: development and comparison of methods. Health Technol Assess 2020; 24: 1–68.
- Hernández Alava M, Wailoo AJ, Ara R. Tails from the peak district: adjusted limited dependent variable mixture models of EQ-5d questionnaire health state utility values. Value Health 2012; 15: 550–561.
- Alava MH, Wailoo A. Fitting adjusted limited dependent variable mixture models to EQ-5D. Stata J 2015; 15: 737–750.
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507.
- Weiner DE, Vervloet MG, Walpen S, Schaufler T, Munera C, Menzaghi F, et al. Safety and effectiveness of difelikefalin in patients with moderate-to-severe pruritus undergoing hemodialysis: an open-label, multicenter study. Kidney Med 2022; 4: 100542.
- Cooper JT, Lloyd A, Sanchez JJG, Sörstadius E, Briggs A, McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes 2020; 18: 310.
- Wang C-C, Tang C-H, Huang K-C, Huang S-Y, Sue Y-M. Increased risk of incident psoriasis in end-stage renal disease patients on chronic hemodialysis: a nationwide population-based cohort study. J Dermatol 2018; 45: 1063–1070.
- Masmoudi A, Hajjaji Darouiche M, Ben Salah H, Ben Hmida M, Turki H. Cutaneous abnormalities in patients with end stage renal failure on chronic hemodialysis. A study of 458 patients. J Dermatol Case Rep 2014; 8: 86–94.
- Brown EA, Zhao J, McCullough K, Fuller DS, Figueiredo AE, Bieber B, et al. Burden of kidney disease, health-related quality of life, and employment among patients receiving peritoneal dialysis and in-center hemodialysis: findings from the DOPPS Program. Am J Kidney Dis 2021; 78: 489–500.e1.
- Agarwal R, Burton J, Gallieni M, Kalantar-Zadeh K, Mayer G, Pollock C, et al. Alleviating symptoms in patients undergoing long-term hemodialysis: a focus on chronic kidney disease-associated pruritus. Clin Kidney J 2022; 16: 30–40.
- Smyth B, van den Broek-Best O, Hong D, Howard K, Rogers K, Zuo L, et al. Varying association of extended hours dialysis with quality of life. Clin J Am Soc Nephrol 2019; 14: 1751–1762.
- Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, et al. Developing a set of core outcomes for trials in hemodialysis: an international delphi survey. Am J Kidney Dis 2017; 70: 464–475.