Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphomas (CTCL) (1, 2). Large cell transformation in MF is defined as the histopathological transformation of neoplastic small-to-medium-sized lymphocytes to a large cell phenotype, often expressing CD30 and accounting for more than 25% of the T-cell infiltrate (3). Large cell transformation occurs in 20–50% of advanced MF, and is generally associated with disease progression and poorer prognosis with a 5-year survival rate of less than 20% (3). Neutrophilic dermatosis (ND) consists of skin lesions for which histological examination reveals intense epidermal, dermal, and/or hypodermal neutrophilic infiltrates with no evidence of infection or true vasculitis. The association of ND with various systemic diseases, including inflammatory bowel disease, rheumatic and various haematological disorders is well documented, but less common, in CTCL (4).
We describe here a case of advanced MF, stage IIB (T3N0M0B0), associated with large cell CD30+ transformation and ND.
CASE REPORT
We report here a case of an 80-year-old woman, initially diagnosed with stage IB MF, who subsequently developed transformed CD30+ disease associated with ND. The first cutaneous manifestations appeared in 2015 and diagnostic of MF was made 2 years later. Initial treatment consisted of topical corticosteroids and psoralen and ultraviolet A (PUVA) therapy. The disease was stable until 2020, when the patient deteriorated, presenting with patches, plaques and tumours, covering more than 70% of the patient’s total body surface. Positron emission tomography – computed tomography (PET-CT) and blood flow cytometry confirmed a skin-limited disease, stage IIB (T3N0M0B0). A treatment with methotrexate (MTX) and interferon-alpha (IFN-α) was initiated. Patient skin condition worsened rapidly and she presented with fever, fierce itching and erythematous and violaceous plaques (Fig. 1a). Plaques evolved to painful, ulcerated skin nodules, along with large pustules (Fig. 1b) not responding to radiotherapy, MTX or systemic corticosteroids. Laboratory parameters demonstrated leukocytosis (16.9 G/L) , including neutrophilia (14.3 G/L), hypereosinophilia (2.5 G/L) and increased immature granulocytes (0.64 G/L), anaemia (101 g/L), thrombocytosis (425 G/L) and high level of lactate dehydrogenase (423 U/L). A new cutaneous biopsy demonstrated a dense, atypical lymphocytic infiltrate and numerous admixed lymphocytes, some medium in size with irregular nuclear outlines and others larger ones showing oval nuclei and pale chromatin (Fig. 1e). Furthermore, there was a dermal infiltrate of polynuclear neutrophils and eosinophils, without oedema in the upper dermis, compatible with neutrophilic dermatosis (Fig. 1f). Epidermis was parakeratotic and infiltrated by atypical cells, neutrophils and eosinophils, which form a few sub-corneal pustules. CD3 and CD4 immunohistochemistry showed CD3+, CD4+ lymphomatous cells (Fig. 1g), 30% of them were CD30+ and corresponded to large cells (Fig. 1h). The histopathological diagnosis of transformed CD30+ MF, stage IIB (T3N0M0B0), with concomitant Sweet-like ND, was established.
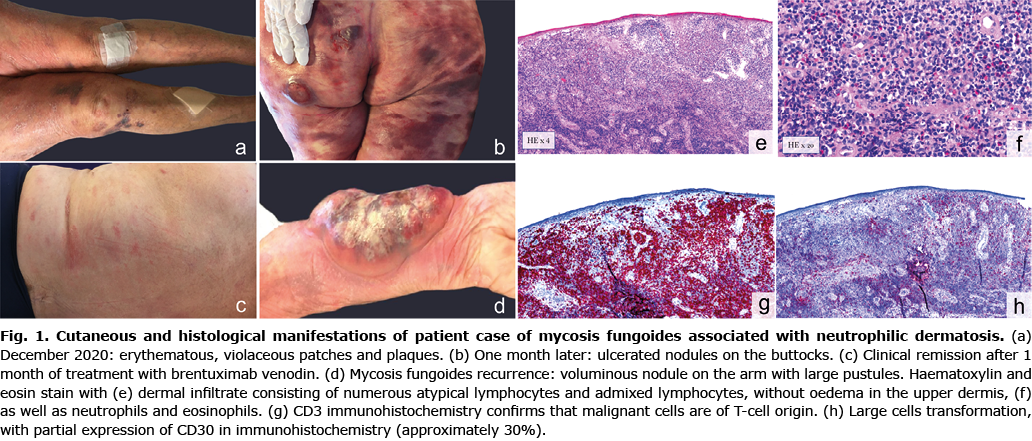
Based on the CD30 positivity, treatment with brentuximab vedotin was initiated and resulted in initial remission over 6 months (Fig. 1c). However, after 6 cycles of brentuximab vedotin, the MF progressed with new ulcerated nodules, pustules (Fig. 1d) and extreme itching. Furthermore, the disease was complicated by a superinfection and sepsis driven by methicillin-resistant Staphylococcus aureus, and later Pseudomonas aeruginosa. Despite a well-conducted antibiotherapy, a further deterioration of the patient general condition was observed. In this context, chemotherapy with doxorubicin was contraindicated, and patient was started on palliative radiotherapy. In the following days, fulminant disease progression was observed and the patient died shortly afterwards.
DISCUSSION
ND may be associated with various systemic diseases, most commonly with myeloid malignancies (5). However, there are only 6 reported cases of MF-associated ND in the literature, and only 2 of them were observed in patients with transformed disease. The types of ND described in these cases vary, including pyoderma gangrenosum, neutrophilic hidradenitis, Sweet’s syndrome and exanthematous pustulosis (6–9). Sweet’s syndrome is the most common type of ND, characterized by fever, painful erythematous plaques with rapid onset, hyperleukocytosis, neutrophilia, massive neutrophilic dermal infiltrate in the histopathology, and a positive response to systemic corticosteroids (10). In the current case, clinical symptoms (fever and painful erythematous plaques) and laboratory parameters (hyperleukocytosis and neutrophilia) were compatible with Sweet’s syndrome. However, oedema in the upper dermis, which is a histological hallmark of Sweet’s syndrome (11), was never present in patient skin biopsies. For this reason, ND in this case was called Sweet-like ND.
The exact pathogenic mechanism of ND development in the current case is unknown, but a plausible role of malignant T-cells has been reported, which in a paracrine manner produce high amounts of interleukin-8 (IL-8) (12). IL-8 induces recruitment of in vitro neutrophils (chemotaxis) and promotes the release of granule enzymes (neutrophil proteases). In human skin, an exclusive infiltration of neutrophils due to IL-8 production has been shown. IL-8 can also be synthetized by neutrophils, which may thus intensify their own recruitment (13).
In all reported cases, including ours, MF associated with ND has a fulminant progression with resistance to usual systemic treatments. Development of ND has been shown to be a poor prognostic factor, regardless of the initial MF staging. All described patients died shortly after the diagnosis of ND (6, 7).
In conclusion, we report a here case of an 80-year-old woman diagnosed with transformed CD30+ MF associated with ND. The patient presented with fulminant MF disease progression with painful skin nodules and fierce itching, despite brentuximab vedotin introduction. She died of sepsis as a disease complication.
The association of MF with ND is rare, under-reported and seems to be related to a poorer prognosis. In such cases, conventional treatments are mainly ineffective and new therapeutic options are needed. ND occurs mainly in advanced MF and could be considered a paraneoplastic process.
The authors have no conflicts of interest to declare.
REFERENCES
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 133: 1703–1714.
- Maguire A, Puelles J, Raboisson P, Chavda R, Gabriel S, Thornton S. Early-stage mycosis fungoides: epidemiology and prognosis. Acta Derm Venereol 2020; 100: adv00013.
- Pulitzer M, Myskowski PL, Horwitz SM, Querfeld C, Connolly B, Li J, et al. Mycosis fungoides with large cell transformation: clinicopathological features and prognostic factors. Pathology (Phila) 2014; 46: 610–616.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol 2000; 18: 355–367.
- Maglie R, Genovese G, Solimani F, Guglielmo A, Pileri A, Portelli F, et al. Immune-mediated dermatoses in patients with haematological malignancies: a comprehensive review. Am J Clin Dermatol 2020; 21: 833–854.
- Morales-Moreno HJ, Montenegro-Damaso T, Peñate Y. Neutrophilic dermatosis associated with mycosis fungoides. JAAD Case Rep 2015; 1: 333–336.
- Franck N, Carlotti A, Gorin I, Buffet M, Mateus C, Dupin N, et al. Mycosis fungoides–type cutaneous t-cell lymphoma and neutrophilic dermatosis. Arch Dermatol 2005; 141: 353–356.
- Aubin F, Dufour MP, Angonin R, Misery L, Laurent R, Humbert P. Sweet’s syndrome associated with cutaneous T cell lymphoma. Eur J Dermatol 1998; 8: 178–179.
- Guillet S, Stokkermans J, Vergier B, Doutre M-S, Beylot-Barry M. Dermatose neutrophilique aiguë pustuleuse au cours d’un mycosis fongoïde transformé agressif. Ann Dermatol Vénéréologie 2013; 140: 635–640.
- Nofal A, Abdelmaksoud A, Amer H, Nofal E, Yosef A, Gharib K, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges 2017; 15: 1081–1088.
- Masmoudi A, Chaaben H, Hamdouni K, Boudaya S, Bouassida S, Turki H, et al. Syndrome de Sweet: Étude rétrospective de 54 cas. Presse Médicale 2007; 36: 419–424.
- Goddard DS, Yamanaka K, Kupper TS, Jones DA. Activation of neutrophils in cutaneous T-cell lymphoma. Clin Cancer Res 2005; 11: 8243–8249.
- Abreu M, Miranda M, Castro M, Fernandes I, Cabral R, Santos AH, et al. IL-31 and IL-8 in cutaneous T-cell lymphoma: looking for their role in itch. Adv Hematol 2021; 2021: 5582581.