ORIGINAL REPORT
Monitoring Sleep and Scratch Improves Quality of Life in Patients with Atopic Dermatitis
Ken-ichi YASUDA1, Yozo ISHIUJI1, Toshiya EBATA1,2, Takamasa KOGURE3, Eitaro KONDO3, Arihito OTA4, Toshihiro ITO5, Koki ENDOH6 and Akihiko ASAHINA1
1Department of Dermatology, The Jikei University School of Medicine, 2Chitofuna Dermatology Clinic, 3Paramount Bed Sleep Research Laboratory, PARAMOUNT BED CO., LTD., 4Department of Dermatology, The Jikei University Daisan Hospital, 5Department of Dermatology, The Jikei University Katsushika Medical Center, Tokyo, and 6Department of Dermatology, The Jikei University Kashiwa Hospital, Chiba, Japan
Atopic dermatitis itch may cause sleep disturbance and impair quality of life. For patients finding topical therapy difficult to continue, it is important to control itch and reduce scratching. This study developed algorithms to measure nocturnal sleep and scratch, using an actigraph device worn on the back of the hand, and assessed smartphone application feedback to improve adherence with therapy. In the first trial, actigraph measurements in 5 participants who wore the device were highly correlated with measurements by a sleep-monitoring device beneath the mattress. Total actigraph-measured scratching duration for each hour of sleep was highly correlated with measurements by a person rating infrared video-recording of the sleepers. In the second trial, 40 patients with atopic dermatitis were randomly allocated into an intervention group that used the actigraph and smartphone application, and a control group that did not. Both groups were instructed to use the same moisturizer. Dermatology Life Quality Index scores decreased significantly from baseline and were lower than those in the control group at week 8. It is suggested that the device and associated smartphone application reinforced therapy adherence, moisturizer use, and contributed to improved quality of life in patients with atopic dermatitis.
Key words: atopic dermatitis; scratching; sleep; quality of life; actigraph.
SIGNIFICANCE
This study reports the development and testing of a device, comprising an actigraph worn on the back of the hand along with a smartphone application, which monitors sleep, nocturnal scratching and adherence in adult patients with atopic dermatitis. The device is expected to be more accurate than existing systems in detecting and evaluating nocturnal scratching behaviour. The study results showed that the newly developed device was reliable and effective to measure sleep and scratching behaviour and that its use with the dedicated smartphone application improved patient’s quality of life.
Citation: Acta Derm Venereol 2023; 103: adv11922. DOI https://doi.org/10.2340/actadv.v103.11922.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 24, 2023; Published: Oct 6, 2023
Corr: Yozo Ishiuji, Department of Dermatology, The Jikei University School of Medicine, 25-8 Nishi-Shimbashi 3-chome, Minato-ku, Tokyo 105-8461, Japan. E-mail: yozoishiuji@gmail.com
Competing interests and funding: KY, YI and AA were funded by NAOS, France, Signtle Inc., and PARAMOUNT BED CO., LTD. TE had an advisory contact with Signtle Inc. at the time of the study. EK and TK are employees of PARAMOUNT BED CO., LTD. The other authors have no conflicts of interest to declare.
INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease characterized by eczema and pruritus, which may affect people of any age (1, 2). Itch is a major symptom of patients with AD, often leading to sleep disturbance, anxiety, and depression, and impairing quality of life (QoL) (3). Patients with AD may experience the itch-scratch cycle, where scratching an itch triggers inflammation causing skin damage inducing itch. Topical application of steroids and moisturizers are effective to improve AD itch when applied appropriately; however, not all patients respond well, due to non-compliance and lack of adherence with therapy. It is essential to promote treatment adherence to control itch and reduce scratching behaviour and encourage patients to use topical drugs to control AD (4). Accurate assessment of itch is important, including at night-time, to inform treatment selection.
Several studies have used subjective evaluation tools to measure itch and sleep disturbance in patients with AD, including the visual analogue scale (VAS) (5), and questionnaires, such as 5-D Itch Scale (6) or Pittsburgh Sleep Quality Index (PSQI) (7, 8). Although there are objective evaluation tools including observation and rating of video-recordings (9) for scratching, and polysomnography (PSG) (10) for sleep, they are labour intensive, and thus expensive and seldom used. On the other hand, wrist-attached actigraphy has often been used in research as a convenient tool for objective evaluation of scratching behaviour and sleep (11–13). However, the use of wrist-attached actigraphy is less common for objective evaluation of scratching than for objective evaluation of sleep (14). Furthermore, wearing wrist-attached actigraphy may be burdensome for some patients with AD, necessitating the development of alternative devices.
This study developed and tested a device that is attached to the dorsum of the hand, which measures sleep and nocturnal scratching using technology that measures movements. For the device to discriminate between scratch and non-scratch hand movements the study devised and tested a set of algorithms. An associated smartphone application (app) was also developed to enable self-monitoring and daily recording of itch, sleep, and the use of topical medications and moisturizers. Daily use of the app, along with monitoring of nocturnal sleep and scratch by the device, is expected to inform patients and reinforce therapy adherence to improve QoL. We describe here development of the algorithms, and testing of the actigraph device in a randomized controlled trial to assess whether use of the device and smartphone app may help patients better control their AD and thereby improve QoL.
MATERIALS AND METHODS
Two clinical trials were conducted. The first (Study 1) was to develop algorithms to detect scratch and sleep and to discriminate between scratch and non-scratch hand movements using a device called a hand actigraph (HA) that measures triaxial acceleration at 25 Hz.
The second (Study 2) was a randomized open-label trial to clarify whether use of the HA and associated smartphone app improved QoL in patients with AD. Users could choose to wear the HA affixed to the dorsum of the hand by adhesive tape (Fig. 1A) or with a cotton “glove” without tape (Fig. 1B).
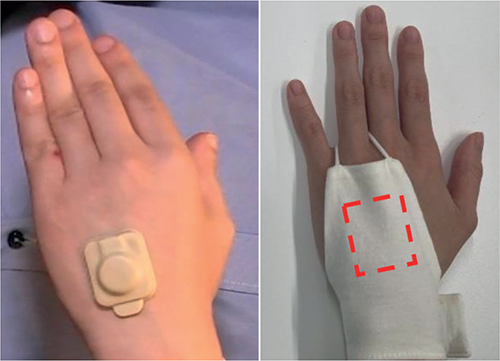
Fig. 1. Actigraph placement. The actigraph was either attached to the dorsum of hand by (a) adhesive or (b) worn in a “glove” at the same location.
Study 1
First, an algorithm to score sleep-wake status during every 1 min was developed by analysing measurements by the HA, worn by 3 healthy participants, and those by a sheet-shaped body vibrometer (SBV; Nemuri SCAN NN-1310, PARAMOUNT BED CO., LTD., Tokyo, Japan). The SBV was placed under the mattress, and detects vibration of a body on the mattress using sensitive pressure sensors. The SBV scores sleep-wake status with similar accuracy to wrist actigraphy (15). The algorithm employed a multiple regression model using activity score. The multiple regression equation for scoring sleep-wake status was derived from a sleep-wake regression equation in which HA scores were the independent variable and SBV scores were the dependent variable.
To discriminate between scratch and non-scratch hand movements, an algorithm was developed using multiple linear regression equations. The actigraph measures movement and acceleration in the direction of all 3 axes. Three multiple regression equations were derived from HA measurements in all directions as independent variables and scores of scratching behaviour rated by an observer of infrared video recordings as the dependent variable. If even 1 value calculated from the equation is more than 0.5, the epoch is classified as scratch.
To further develop and test the sleep and scratch scoring algorithms, 7 participants with AD were recruited for simultaneous measurement of HA and SBV. Data for 2 participants was excluded due to missing accelerometer data because of dislodged HA, leaving data for 5 (3 male, 2 female, mean ± standard deviation age 35 ± 7.9 years) for analysis. All participants were examined by 1 experienced dermatologist who rated their AD severity as moderate to severe (EASI 18.4 ± 6.1). Their self-evaluated itch severity for the previous 24 h was 7.2 ± 1.2 on the 11-point Pruritus Numeric Rating Scale (NRS, 0 = no itch, 10 = worst imaginable itch). While being recorded by an infrared camera, participants wore an HA and slept under the same conditions of SBV and bedding overnight for a minimum 7 h in an air-conditioned room. To facilitate evaluation of scratching in the infrared recordings, which was scored by the same observer, the cover blanket was thin. In the recordings of movements during sleep, the evaluator identified scratching episodes and documented data including episode time, length, and which hand was involved.
Study 2
To enable patients with AD to record and self-monitor behaviour to inform and reinforce adherence with prescribed treatments, the app included 3 functions: display HA scores of scratch bouts and sleep status; record Dermatology Life Quality Index (DLQI) and PSQI once a week, Itch NRS and Sleep Numeric Rating Scale (Sleep NRS: 0 = worst-, 10 = best-possible-sleep) in the previous past 24 h; and remind and record use of medications and moisturizers.
Study 2 was conducted in the Department of Dermatology, Jikei University School of Medicine; Jikei University Daisan Hospital; Jikei University Katsushika Medical Center; and Jikei University Kashiwa Hospital. Forty patients with AD were recruited who met the following inclusion criteria: (i) over 20 years; (ii) fulfilment of AD-diagnostic criteria of Hanifin and Rajka (16), (iii) mild to severe AD according to severity criteria; 1.1~50.0 score in EASI, (iv) patients with moderately or severely impaired QoL; ≥ 6 score in DLQI, (v); able to understand and cooperate with written consent, study instructions, visits, and procedure (Table I). They were randomly assigned into intervention and control groups by block randomization (Fig. 2). All patients attended hospital on the first day (0 week), and at 4-weeks, and 8-weeks. DLQI, EASI, Patient-Oriented Eczema Measure (POEM), PSQI, and Itch NRS in the previous 3 days were assessed on each day of their visit. All patients continued their medical treatment for AD, such as topical corticosteroids and oral antihistamines, throughout the study period. However, for all subjects, the skin moisturizer was restricted to the same dermocosmetic, Atoderm® Intensive cream (Laboratoire Bioderma, France) (17). The amount of moisturizer used was measured to evaluate therapy adherence. The primary endpoint was improved DLQI score, with secondary endpoints being improved scores of Itch NRS in the last 3 days, POEM, PSQI, EASI, and amount of moisturizer used.
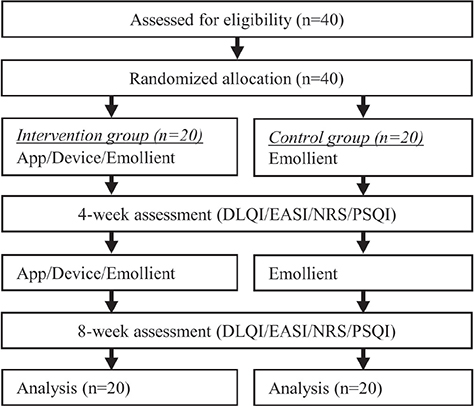
Fig. 2. Flowchart of study 2. DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; NRS: Itch Numerical Rating Scale; PSQI: Pittsburgh Sleep Quality Index.
Statistical analyses
Statistical analysis was performed using R (version 4.3.0) with p < 0.05 deemed significant. Linear mixed-effects (LME) analysis using lme4 package was performed for repeated measures: DLQI, EASI, NRS and PSQI. The target variable was raw score of measurements. Candidates of fixed effects were Group (2 levels: Intervention, Control), Time (3 levels: 0 weeks, 4 w, 8 w) and interaction factor. Identification Number of the patients was included as a random variable. The model with lowest Akaike Information Criteria was selected as a best fit model. Post-hoc multiple comparisons of least square means from the selected model were adjusted by Bonferroni correction. A χ2 test was used to compare clinically important differences among the studied groups.
Ethical considerations
Study 1 protocol was approved by the Institutional Review Board of Kanazawabunko Hospital ethics committee, and that for Study 2 by Jikei University School of Medicine (approval number 10135). All participants provided written informed consent. The study accorded to the tenets of the Declaration of Helsinki.
RESULTS
Study 1
For HA, to score sleep in a 1-min period, the length of a continuous time window was optimized, and 5 continuous minutes targeting a central minute was chosen. The acquired equation is given below:
Dt = 0.0001(16 At-2 + 12 At-1 + 32 At + 10 At + 1+10 At + 2
where Dt is the calculated value in a target minute and At, At-1, At+1, etc. represent the activity count in the target minute, the previous minute, the following minute, etc. If Dt was lower than or equal to 1, it was classified as sleep, otherwise it was classified as awake.
Sleep status by HA scored an agreement rate of 90.5% (2,242/2,476 epochs), sensitivity of 95.5% (1,985/2,079 epochs), and specificity of 64.7% (257/397 epochs) compared with SBV score in 5 participants with AD.
To score scratching, the acquired equations are shown in Fig. 3. Total HA-recorded scratching duration in each hour of sleep (estimated scratch length) was significantly positively correlated with total infrared recorded evaluation of scratching in the same period with the left hand (r = 0.897, slope = 1.12), and both hands (r = 0.754, slope = 2.31) (Fig. 4).
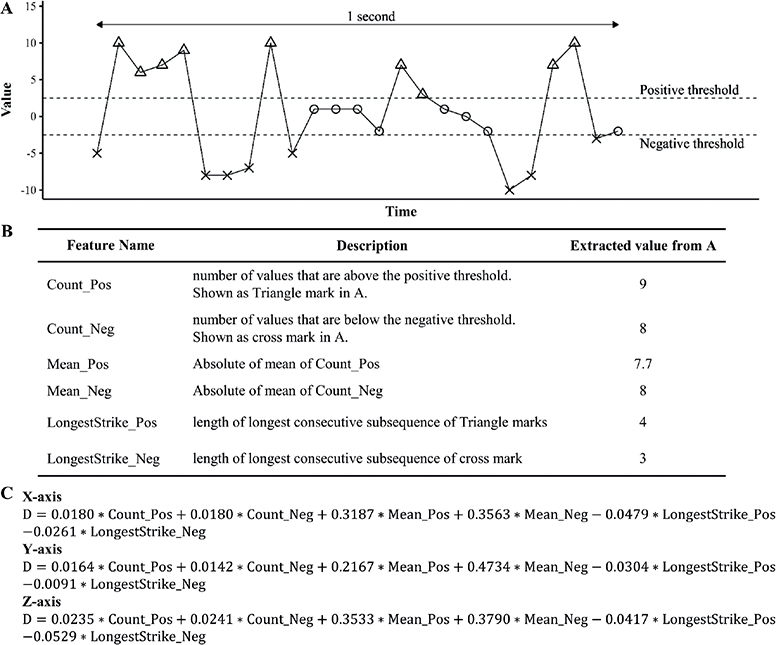
Fig. 3. Feature extraction and equations to score scratch. (A) The graph shows conceptual acceleration data captured in 1 s from 1 of the 3 axes. Triangle, circle and cross marks represent data points. (B) List of extracted features. (C) List of acquired equations. D is calculated value. x-axis is the fingers to wrist direction, y-axis is the thumb to little finger direction, and z-axis the dorsum of hand to palm direction.
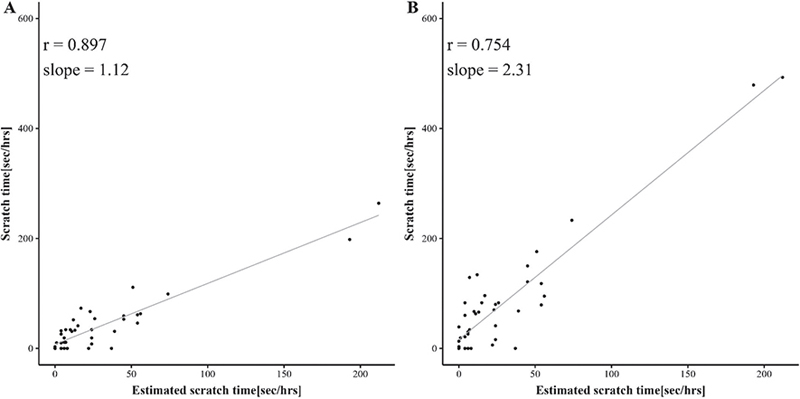
Fig. 4. The total device-recorded scratching duration in each hourly section of sleep in Study 1. (A) Estimated scratch time vs evaluator recorded scratching by the left hand. (B) Estimated scratch time vs evaluator-recorded scratching by both hands.
Study 2
Fig. 5 shows scores of repeated measures of clinical outcomes. From linear mixed-effects (LME) analysis of DLQI, the full model was selected as the best model (Table SI). Multiple comparisons using the full model showed a significant difference between the intervention and control group at week 8 in DLQI score. DLQI score in the intervention group was significantly decreased at weeks 4 and 8 compared with week 0 (Fig. 5). Defining clinically important improvement as a decrease in DLQI of ≥ 4 from 0 to 8 weeks (18) or a decrease in absolute score to < 1 point at 8 weeks, the proportion of patients who achieved significant improvement in the intervention group at 8 weeks (12/20 60%) was double that in the control group (7/20 35%); however, this was not statistically significant by χ2 test (p = 0.2053). In the analysis of EASI, POEM, and Itch NRS using LME, the analysis did not include the interaction factor, but included the Time factor (Table SI). The best model indicated that the scores observed at week 0 significantly decreased by week 8 in EASI, POEM, and Itch NRS (Table SII). However, for PSQI, we only selected the null model (intercept model), and we did not perform multiple comparisons.
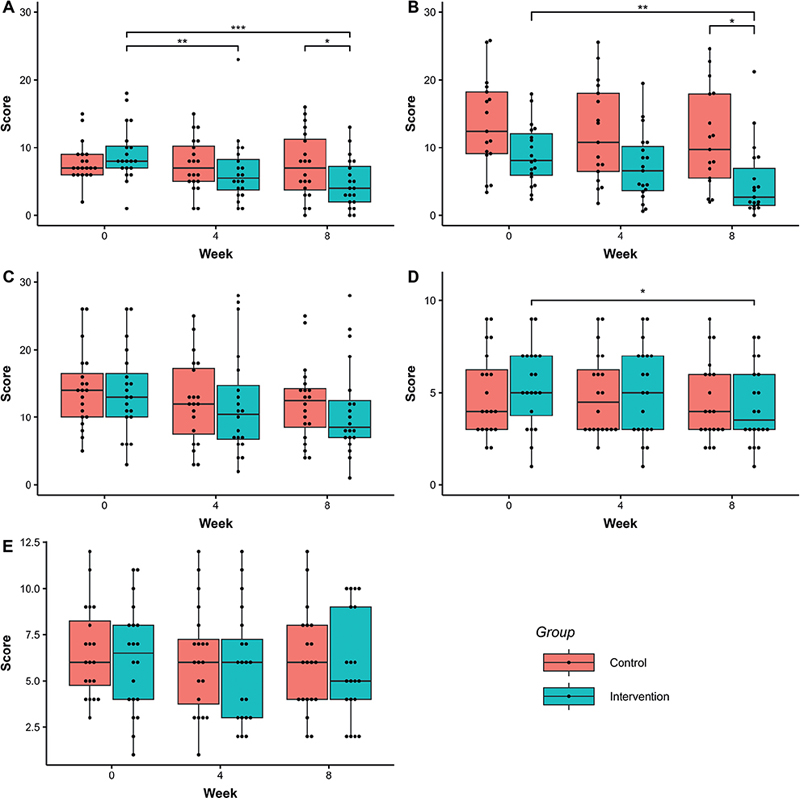
Fig. 5. Change in Dermatology Life Quality Index (DLQI), Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), Itch Numerical Rating Scale (NRS) and Pittsburgh Sleep Quality Index (PSQI) scores in Study 2. (A) DLQI, (B) EASI, (C) POEM, (D) NRS, (E) PSQI. Each dot represents observed values. Significant codes: ***p < 0.001,’**p < 0.01, *p < 0.05.
The amount of moisturizer use in both the intervention and control groups was measured, and although the difference was not statistically significant, the intervention group had a higher mean use (355.3 ± 239.8 g) compared with control group (280.0 ± 267.2 g, p = 0.372). The study measured total sleep time, sleep efficiency, scratch time, and the percentage of total scratch time (scratch time/total sleep time) using the HA. LME analysis was performed to detect changes in these 4 HA measurements over time. In all cases, the null model was the best compared with the model with the time factor (Table SIII).
DISCUSSION
The HA device enabled the measurement of nocturnal scratch and sleep in patients with AD, and the associated smartphone app recorded these measurements. LME analysis revealed a significant improvement in DLQI score in the intervention group compared with the control group. This suggests that the use of the HA device and app contributed to improved QoL.
While secondary endpoints, such as EASI, POEM, and Itch NRS, all showed time-dependent improvements, with greater mean improvements in the intervention group compared with the control group, we did not find a significant difference between the 2 groups, unlike with DLQI score. One possible explanation for the improvements in EASI, POEM, and Itch NRS in both groups could be the concurrent use of the medical treatments and/or the application of a novel moisturizer emollient. The emollient contains ingredients, such as vitamin B3 palmitoylethanolamide (PEA), known for its antipruritic properties, sucroesters, β-sitosterol, which are anti-inflammatory agents, and zinc, which has its antibacterial properties against Staphylococcus aureus known to trigger exacerbation of AD (17). Preliminary unpublished studies have shown that new emollient containing vitamin B3 helps to restore and strengthen natural skin barrier function by inducing the synthesis of epidermal lipids. Another possibility for the improvements in both groups could be participation in the study itself. Being involved in the study may have motivated the patients with AD to adhere more diligently to their treatment with regimen, resulting in better outcomes. Even patients in the control group, who did not use a sham device or sham app, had their moisturizer volume measured, which could have increased their conscious use of the moisturizer, contributing to improvements in their symptoms. This could lead to the improvement in EASI, POEM, and NRS in both groups. Furthermore, patients in the intervention group might have avoided aggravating factors for atopy, such as alcohol consumption and stress, as they were being monitored by the device during the night. However, these aggravating factors were not assessed in this research. No significant improvements in sleep quality or nocturnal scratching were observed. This suggests that sleep quality, including nocturnal scratching, may not have contributed to the overall improvement in QoL. This may be due to the fact that the PSQI scores (where lower scores indicate better sleep quality) were only slightly elevated at the beginning of the study on day 0, and sleep efficiency was already high, averaging 90% in the first week.
The activity score multiple regression model is commonly used for scoring sleep (19, 20). Although the number of participants in the current study was not sufficient to draw definitive conclusions, it was found that the HA device showed high performance, in scoring scratching and sleep in patients with AD. We believe that the performance of the HA is clinically sufficient and acceptable. Ikoma et al. (13) previously reported measurements of nocturnal scratching using an Apple watch (Apple Inc., Cupertino, CA, USA), a popular consumer sleep tracker, in both laboratory and real-life settings. However, Chun et al. (21) suggested that a wireless sensor placed on the back of the hand could provide more accurate measurements, particularly for finger-only scratching more accurately. In the current study, the HA measured sleep disturbance and scratching simultaneously, which is an important advantage, since these factors are closely related to QoL.
Study 2 revealed that the combination of the HA device, the app, and the moisturizer led to improved QoL in patients with AD, although there were no significant improvements in clinical symptoms and patient-reported outcomes compared with the control group. The underlying mechanism behind the improvement in QoL remains unclear. However, we hypothesize that the use of the HA device and app to monitor the disease status helped the patients to maintain continuous awareness of their condition and apply ointment properly, thereby contributing to the improvement in QOL. Using SBV as a reference for sleep instead of PSG and the small sample size are limitations. Therefore, we employed a simple and traditional algorithm for sleep and scratch scoring.
In conclusion, this research suggests that the simultaneous use of the HA, the app, and the moisturizer used in this study may contribute to the improvement in QoL in patients with AD.
ACKNOWLEDGEMENTS
IRB approval status: The protocol for Study 1 was approved by the ethics committee of Kanazawabunko Hospital IRB, and for Study 2 by that of Jikei University School of Medicine (approval number 10135). All participants provided written informed consent.
REFERENCES
- Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494.
- Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy 2014; 2014: 354250.
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018; 121: 340–347.
- Tier HL, Balogh EA, Bashyam AM, Fleischer AB Jr, Spergel JM, Masicampo EJ, et al. Tolerability of and adherence to topical treatments in atopic dermatitis: a narrative review. Dermatol Ther 2021; 11: 415–431.
- Ibáñez MD, Rodríguez Del Río P, González-Segura Alsina D, Villegas Iglesias V. Effect of synbiotic supplementation on children with atopic dermatitis: an observational prospective study. Eur J Pediatr 2018; 177: 1851–1858.
- Udkoff J, Silverberg JI. Validation of scratching severity as an objective assessment for itch. J Invest Dermatol 2018; 138: 1062–1068.
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213.
- Kaaz K, Szepietowski J, Matusiak Ł. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol 2019; 99: 175–180.
- Ebata T, Aizawa H, Kamide R, Niimura M. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol 1999; 141: 82–86.
- Chang YS, Chiang B-L. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol 2018; 142: 1033–1040.
- Bender BG, Leung SB, Leung DYM. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allerg Clin Immunol 2003; 111: 598–602.
- Chang YS, Chou YT, Lee JH, Lee PL, Dai YS, Sun C, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 2014; 134: e397–405.
- Ikoma A, Ebata T, Chantalat L, Takemura K, Mizzi F, Poncet M, et al. Measurement of nocturnal scratching in patients with pruritus using a smartwatch: Initial clinical studies with the itch tracker app. Acta Derm Venereol 2019; 99: 268–273.
- Yang AF, Nguyen M, Li AW, Lee B, Chun KS, Wu E, et al. Use of technology for the objective evaluation of scratching behavior: a systematic review. JAAD Int 2021; 5: 19–32.
- Kogure T, Shirakawa S, Shimokawa M, Hosokawa Y. Automatic sleep/wake scoring from body motion in bed: validation of a newly developed sensor placed under a mattress. J Physiol Anthropol 2011; 30: 103–109.
- Jon M. Hanifin GR. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; 92: 44–47.
- Gayraud F, Sayag M, Jourdan E. Efficacy and tolerance assessment of a new type of dermocosmetic in infants and children with moderate atopic dermatitis. J Cosmet Dermatol 2015; 14: 107–112.
- Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology 2015; 230: 27–33.
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep 1992; 15: 461–469.
- Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 2001; 105: 185–191.
- Chun KS, Kang YJ, Lee JY, Nguyen M, Lee B, Lee R, et al. A skin-conformable wireless sensor to objectively quantify symptoms of pruritus. Sci Adv 2021; 7: eabf9405.