ORIGINAL REPORT
Evaluation of the Diagnostic Value of Oesophageal Biopsies for Direct Immunofluorescence Microscopy in Mucous Membrane Pemphigoid
Kaan YILMAZ1,2, Onur DIKMEN1,3, Nina VAN BEEK1, Jens U. MARQUARDT4, Martha M. KIRSTEIN4, Detlef ZILLIKENS1 and Enno SCHMIDT1,5
1Department of Dermatology, University of Lübeck, Lübeck, 2Department of Dermatology, University of Heidelberg, Medical Faculty Mannheim, Mannheim, 3Charité – Universitätsmedizin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Institute of Pathology, Berlin, 4Department of Medicine I, University of Lübeck, Lübeck and 5Lübeck Institute of Experimental Dermatology (LIED), University of Lübeck, Lübeck, Germany
Mucous membrane pemphigoid is an autoimmune blistering disorder characterized by predominant involvement of surface-close epithelia and linear depositions of immunoreactants at the dermal-epithelial junction on direct immunofluorescence microscopy. A major diagnostic difficulty is the frequent need for multiple biopsies to facilitate the diagnosis. Although oesophageal involvement is a rare, but life-threatening manifestation, the relevance of oesophageal direct immunofluorescence sampling is unclear. This retrospective monocentric study evaluated 67 non-lesional biopsies from 11 patients with mucous membrane pemphigoid and clinical symptoms suggestive of oesophageal involvement, comprising 31 samples from the oesophagus and 36 samples from other anatomical sites. Five patients (45.5%) exhibited endoscopic findings compatible with oesophageal involvement of mucous membrane pemphigoid. No correlation was identified between the presence of oesophageal lesions and direct immunofluorescence positivity in lesions from the oesophagus (p = 1.0). Oral and cutaneous samples were significantly more frequently positive by direct immunofluorescence than were oesophageal biopsies (p < 0.0001 and p = 0.0195, respectively). Oesophageal samples yielded significantly less IgG reactivity than oral and cutaneous lesions (p < 0.0001 and p = 0.0126, respectively), and less IgA antibody response than oral lesions (p = 0.0036). In conclusion, oesophageal direct immunofluorescence samples were inferior to oral and cutaneous biopsies for the diagnosis of mucous membrane pemphigoid even when oesophageal lesions compatible with mucous membrane pemphigoid were present at the time of biopsy.
Key words: mucous membrane pemphigoid; autoimmune bullous diseases; autoantibody; direct immunofluorescence microscopy; oesophagus; dysphagia.
SIGNIFICANCE
The diagnostic gold standard for mucous membrane pemphigoid, a potentially fatal autoimmune blistering disease, is direct immunofluorescence microscopy of a perilesional biopsy. The aim of this study was to assess the diagnostic accuracy and clinical-immunopathological correlations of biopsies obtained from the oesophagus, as its epithelium represents a possible target of autoantibodies in mucous membrane pemphigoid. The results suggest that findings from direct immunofluorescence microscopy of oesophageal biopsies do not correlate with endoscopic findings and symptoms indicative for oesophageal involvement. Thus, these data favour oral or cutaneous perilesional biopsies for direct immunofluorescence microscopy even in the presence of oesophageal lesions and/or symptoms compatible with mucous membrane pemphigoid.
Citation: Acta Derm Venereol 2023; 103: adv11947. DOI https://doi.org/10.2340/actadv.v103.11947.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 29, 2023; Published: Aug 25, 2023
Corr: Enno Schmidt, Department of Dermatology, University of Lübeck, Lübeck, Germany, Ratzeburger Allee 160, DE-23562 Lübeck, Germany. E-mail: enno.schmidt@uksh.de
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Mucous membrane pemphigoid (MMP) is a subepithelial autoimmune blistering disease defined by autoantibodies against structural proteins of dermal-epithelial junction (DEJ) and predominant mucosal involvement (1). Antigenic targets of IgG, and less frequently, IgA autoantibodies, include BP180 (type XVII collagen), laminin 332, BP230 (in conjunction with anti-BP180 reactivity), type VII collagen, and possibly α6β4 integrin (2). Rarely, exclusive IgM reactivity has been described in MMP (3, 4). MMP represents a clinically and immunologically heterogeneous disorder potentially affecting several mucosal surfaces, e.g. oral cavity, conjunctivae, nostrils, larynx, pharynx, and anogenital area (5, 6). Oesophageal involvement is rare and may occur in 5–15% of patients (7). It may lead to severe strictures and stenosis, resulting in malnutrition, or even growth retardation in adolescent patients (8). Clinical symptoms of oesophageal involvement in MMP range from oral pain to dysphagia, odynophagia, and weight loss. Such MMP patients are generally recommended to undergo diagnostic oesophagogastroduodenoscopy (OGD) in order to establish or exclude oesophageal involvement (9).
The gold standard diagnostic procedure for MMP is direct immunofluorescence microscopy (DIF) of a perilesional skin and/or mucosal biopsy in which linear deposits of IgG, IgA, IgM and/or complement C3 can be detected at the DEJ (5). A serious drawback, however, is the relatively low sensitivity of 50–70% in the initial biopsy for the diagnosis of MMP, which might entail multiple and repeated sampling (10, 11). To date, there is no consensus regarding if, and to what extent, oesophageal biopsies should be obtained as part of the routine diagnostic work-up in patients with clinical findings suggestive of MMP (2, 11). In previous studies, the diagnostic relevance of oesophageal DIF sampling in MMP has not yet been addressed systematically (8, 12, 13).
MATERIALS AND METHODS
Study cohort
This retrospective study included 11 patients with MMP who underwent OGD with concomitant oesophageal DIF sampling for diagnostic purposes, independent of pretreatment status. All biopsies were evaluated in the routine autoimmune diagnostic laboratory of the University of Lübeck, Germany, between 2006 and 2021. Diagnosis of MMP was made in accordance with current guidelines and based on: (i) clinical findings with predominant mucosal involvement, (ii) linear depositions of IgG and/or IgA and/or C3 at the DEJ by DIF, and/or (iii) detection of circulating autoantibodies against basement membrane zone (BMZ) by (a) indirect immunofluorescence microscopy (IIF) on monkey oesophagus and/or human salt-split skin, (b) enzyme-linked immunosorbent assay (ELISA) for IgG serum reactivity against BP180 NC16A, BP230, or type VII collagen (all Euroimmun, Lübeck, Germany), or (c) immunoblotting with concentrated conditioned medium of cultured HaCaT cells (for IgG and IgA against LAD-1, the soluble ectodomain of BP180), a C-terminal fragment of BP180 (BP180(ec)3, amino acids 1022 to 1266), or extracellular matrix of cultured HaCaT cells (for IgG4 reactivity against laminin 332) (11, 14–17).
Clinical and immunopathological data
Parameters included in this study, i.e. age, sex, clinical and endoscopic findings and immunopathological data, were retrieved from electronic medical records at the Department of Dermatology, University of Lübeck, Lübeck, Germany. Oesophageal involvement was established by OGD findings, as reported previously (9). Pathological oesophageal alterations suggestive of MMP were classified into active, i.e. erythema, blisters, erosions, ulcerations, and cicatricial lesions (i.e. strictures, fibrosis, stenosis, scarring with web formation), as described previously (8).
Statistical analysis
Fisher’s exact test was used to compare 2 different groups. A p - value < 0.05 was considered statistically significant. Statistical analyses were performed, and graphs were made using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Demographic profile of the study cohort
Four female and 7 male patients were included. The mean age (± standard deviation; SD) at diagnosis was 77.3 (± 7.1) years. The median age of study participants was 80 years, ranging from 67 to 88 years (Table I).
Clinical features of the study population
All patients showed lesions within the oral cavity (100%), followed by ocular (in 8 patients), oesophageal (in 5 patients), pharyngeal (in 5 patients), laryngeal (in 4 patients) and nasal mucosae (in 3 patients) as well as the skin (in 3 patients). No involvement of the anorectal and genital mucous membranes was observed (Table I).
Oral pain was the most frequently reported symptom (in 10 patients). Dysphagia was present in 5 patients, occasionally accompanied by odynophagia, coughing, globus and dysgeusia (in 1 patient each) (Table SI). Unintentional weight loss occurred in 2 out of 3 patients who provided information on their weight status (Table I).
All patients underwent oesophageal biopsies during OGD performed due to their indicative symptoms. Five of these patients were under systemic immunosuppressive and/or immunomodulatory therapy at the time of OGD (Table SI). Overall, 5 patients showed macroscopic oesophageal findings compatible with MMP (Table SI). Within this group, 2 patients demonstrated exclusively active lesions (erythema, blisters, erosions and/or ulcerations), whereas solely cicatricial changes (strictures, fibrosis, stenosis, scarring with web formation) were detected in 1 patient. Concomitant presence of active and cicatricial lesions was identified in 2 patients (Fig. 1, Table I).
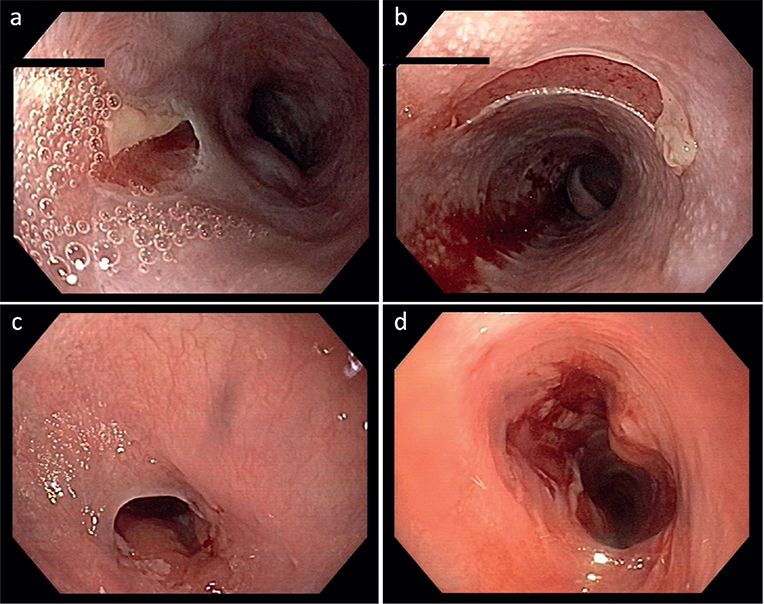
Fig. 1. Endoscopic images of the oesophagus showing (a, b) erosions of the mucous membrane in patient 7 and (c, d) demonstrating a stricture of the proximal oesophagus prior and following dilatation up to 14 mm in patient 6. Macroscopically, the findings were compatible with oesophageal involvement of mucous membrane pemphigoid, which was confirmed by direct immunofluorescence microscopy in both cases.
Immunopathological aspects and evaluation of subsite-specific sampling
A cohort analysis of immunopathological characteristics was performed in patients with MMP and clinical symptoms suggestive of oesophageal affection. Of note, evaluation of DIF samples obtained from the oesophagus yielded no correlation with clinical oesophageal involvement (p = 1.000; OR 1.250; 95% confidence interval (95% CI) 0.05811–26.89) (Table II). No significant difference with regard to oesophageal DIF positivity was highlighted between patients with vs without systemic therapy at the time of OGD (p = 1.000; OR 0.5769; 95% CI 0.03728–5.494). Subsequently, pooled analysis of all 67 biopsies obtained from 11 patients with MMP was conducted, including, inter alia, 31 oesophageal, 16 oral, and 11 cutaneous biopsies. Overall, perilesional oral biopsies showed significantly more positive DIF results than oesophageal biopsies in all patients with MMP (p < 0.0001; OR 0.03571; 95% CI 0.008907–0.1847). A parallel, but less pronounced, tendency was observed for cutaneous biopsies in comparison with oesophageal samples (p = 0.0195; OR 0.01286; 95% CI 0.02393–0.6909) (Table III). In this regard, DIF positivity was identified in 5 skin biopsies obtained from 4 patients, of which 2 had cutaneous involvement of MMP (Table SI). On the other hand, no significant difference was found in terms of DIF positivity between oral and cutaneous biopsies in this study population (p = 0.2238; OR 0.2778; 95% CI 0.05387–1.432) (Table III).
Subsite-specific distribution of in vivo bound immunoreactants by DIF revealed a significantly higher prevalence of IgG deposits in oral as well as cutaneous biopsies compared with oesophageal samples (p < 0.0001; OR 50.00; 95% CI 5.348–467.4, and p = 0.0126; OR 17.4; 95% CI 1.649–178.2, respectively). Oral samples also showed significantly more IgA depositions than biopsies obtained from the oesophagus (p = 0.0036; OR 9.333; 95% CI 1.996–43.64). On the contrary, no significant difference was seen between cutaneous and oesophageal samples with regards to IgA deposits (p = 0.0635; OR 5.333; 95% CI 0.9634 – 29.52) (Fig. 2).
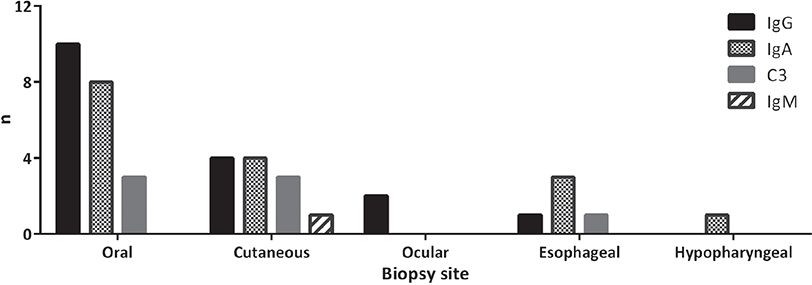
Fig. 2. Site-specific distribution of tissue-bound autoantibodies in direct immunofluorescence microscopy, based on pooled analysis of all samples from 11 patients with mucous membrane pemphigoid (MMP). Total number of biopsies from oral cavity (n = 16), skin (n = 11), eyes (n = 3), oesophagus (n = 31), hypopharynx (n = 2). Ig: immunoglobulin.
Serological studies revealed IgG autoantibodies against the 16th non-collagenous domain (NC16A) of BP180 in 2 patients with oesophageal involvement. Similarly, the soluble ectodomain of BP180 (LAD-1) was recognized by IgG and IgA autoantibodies in 2 patients. IgG autoantibodies recognizing a C-terminal stretch of BP180 (BP180(ec)3), and laminin 332 were found in 1 patient each. Autoantibodies against BP230 were not detected in this cohort, while BP230 was the most frequently targeted epitope in patients without oesophageal affection (in 2 patients), followed by BP180 NC16A (in 1 patient) and a C-terminal domain of BP180 (BP180 4575) (in 1 patient) (Table II).
DISCUSSION
Detection of tissue-bound autoantibodies against BMZ by DIF is regarded as the major diagnostic criterion of MMP and other pemphigoid diseases (1, 5). Reports regarding the sensitivity of DIF in MMP have been inconclusive, demonstrating a wide range from 41% to 100% for mucosal biopsies (10, 18–21), and 44% to 100% for skin samples (10, 18, 20, 21). Notably, in previous studies, circulating autoantibodies have been detected in only 2.6% to 8% of patients with MMP using monkey oesophagus substrate (22–24), and in 36% to 84% with 1 M sodium chloride-split normal human skin (15, 23, 25–29). Owing to the comparably low sensitivity of DIF and IIF (11), the definitive diagnosis of MMP has posed a core challenge. As demonstrated by Shimanovich et al. (10), multiple and repeated sampling may be required to increase the sensitivity of DIF in MMP.
In this retrospective study, only 3 out of 31 biopsies obtained from oesophagus (9.6%) showed linear depositions of immunoreactants (Table III). This corroborates with previous findings by Zehou et al. (8), in which all oesophageal DIF samples from 5 patients with MMP and established oesophageal involvement were negative or not interpretable. In contrast, a study reported 5 patients with MMP who had endoscopic findings compatible with oesophageal involvement, and positive DIF staining of perilesional oesophageal biopsies (12). However, this can be attributed to its selection criteria, as only patients with positive oesophageal DIF results were included. Of note, that study found a heterogeneous anti-BMZ response for IgA and, to a lesser extent, for IgG in MMP with oesophageal involvement. This concurs well with the current data regarding an IgA-predominant mixed autoantibody response in positive oesophageal DIF samples (Fig. 2). In this context, a dual circulating autoreactivity of IgA and IgG has been found to be associated with a more severe disease profile in MMP than an exclusively IgG-mediated disease (29). Remarkably, the current study could identify antigenic targets in all 5 patients with affection of the oesophagus, whilst autoantigens remained elusive in half of the patients without oesophageal alterations (Table II). This could possibly be attributed to higher levels of autoantibodies in the former cohort, leading to severe disease phenotype with oesophageal involvement, whereas low serum antibody levels in the latter group might remain undetectable. Anti-LAD-1 IgG and IgA autoantibodies as well as anti-laminin 332 IgG autoantibodies were found in 2, 2, and 1 patient(s) with oesophageal involvement, respectively, but in none of the patients in the other cohort. Conversely, BP230 was recognized by autoantibodies in one-third of the patients lacking oesophageal lesions, while being absent in patients exhibiting those (Table II). Due to the small sample size, no definite association can be determined between a specific target antigen and oesophageal involvement in MMP.
The optimal biopsy location for DIF assay in MMP has been a point of controversy for many years. Recent evidence suggests that punch biopsy from uninvolved buccal mucosa is as sensitive as perilesional cutaneous biopsy, and superior to gingival biopsies (30). The current study adds to a growing body of research on this issue, demonstrating that oral and cutaneous biopsies led to a significantly higher rate of positive DIF results in patients with MMP compared with oesophageal biopsies. Notably, in the current study, tissue-bound autoantibodies were detected only in the proximal third of the oesophagus (Table III). This is in line with a previous study, which demonstrated that active oesophageal lesions were found predominantly in the upper third segment in patients with MMP (8). This predilection may be due to the transition from stratified squamous epithelium in the upper oesophagus, resembling that in the oral mucosa, to single-layered columnar epithelia in the lower parts. However, further investigations are needed to estimate the expression levels of structural DEJ proteins in different parts of the oesophagus.
Oesophageal involvement is considered one of the most life-threatening, “high-risk” manifestations of MMP, affecting approximately 5–15% of patients with MMP (1, 7, 8, 31). Several experts have recommended endoscopic examination in symptomatic patients to allow early diagnosis and prevent irreversible complications (8, 9). In this study, no correlation was found between oesophageal involvement in MMP and DIF results of oesophageal samples. Future studies are required to validate these results by a larger sample size. Since oesophageal biopsies are not performed on a regular basis in patients with MMP, a multicentric approach could provide an extended patient cohort.
In conclusion, the current data suggest that oesophageal samples are inferior to oral and cutaneous biopsies in the diagnosis of MMP. Therefore, oesophageal biopsies should be performed only after careful benefit-risk assessment.
ACKNOWLEDGEMENTS
This study received infrastructural and financial support from the Deutsche Forschungsgemeinschaft (DFG) by the Collaborative Research Center (CRC) 1526 Pathomechanisms of Antibody-mediated Autoimmunity (PANTAU), grant no. 454193335.
IRB approval status: The study adheres to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Lübeck (22-074).
REFERENCES
- Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002; 138: 370–379.
- Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev 2022; 21: 103036.
- Yilmaz K, Hammers CM, Boch K, Zillikens D, Shimanovich I, Schmidt E. Immunoglobulin M mucous membrane pemphigoid. J Dtsch Dermatol Ges 2023; 21: 285–287.
- Tazudeen N, Au S, Pewitt J, Tu E, Aronson IK. IgM ocular cicatricial pemphigoid: a unique insight into the immune system. Dermatol Online J 2015; 21: 13030/qt40z314gz.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet 2013; 381: 320–332.
- van Beek N, Kridin K, Buhler E, Kochan AS, Stander S, Ludwig RJ, et al. Evaluation of site- and autoantigen-specific characteristics of mucous membrane pemphigoid. JAMA Dermatol 2022; 158: 84–89.
- Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry RJ, Caproni M, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology – Part I. J Eur Acad Dermatol Venereol 2021; 35: 1750–1764.
- Zehou O, Raynaud JJ, Le Roux-Villet C, Alexandre M, Airinei G, Pascal F, et al. Oesophageal involvement in 26 consecutive patients with mucous membrane pemphigoid. Br J Dermatol 2017; 177: 1074–1085.
- Benoit S, Scheurlen M, Goebeler M, Stoevesandt J. Structured diagnostic approach and risk assessment in mucous membrane pemphigoid with oesophageal involvement. Acta Derm Venereol 2018; 98: 660–666.
- Shimanovich I, Nitz JM, Zillikens D. Multiple and repeated sampling increases the sensitivity of direct immunofluorescence testing for the diagnosis of mucous membrane pemphigoid. J Am Acad Dermatol 2017; 77: 700–705.e3.
- Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology – Part II. J Eur Acad Dermatol Venereol 2021; 35: 1926–1948.
- Egan CA, Hanif N, Taylor TB, Meyer LJ, Petersen MJ, Zone JJ. Characterization of the antibody response in oesophageal cicatricial pemphigoid. Br J Dermatol 1999; 140: 859–864.
- Schattenkirchner S, Lémann M, Prost C, Caux F, Guigui B, Cadot M, et al. Localized epidermolysis bullosa acquisita of the esophagus in a patient with Crohn’s disease. Am J Gastroenterol 1996; 91: 1657–1659.
- Hofmann SC, Günther C, Böckle BC, Didona D, Ehrchen J, Gaskins M, et al. S2k Guideline for the diagnosis and treatment of mucous membrane pemphigoid. J Dtsch Dermatol Ges 2022; 20: 1530–1550.
- Schmidt E, Skrobek C, Kromminga A, Hashimoto T, Messer G, Bröcker EB, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol 2001; 145: 778–783.
- Goletz S, Probst C, Komorowski L, Schlumberger W, Fechner K, van Beek N, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol 2019; 180: 149–156.
- Boch K, Hammers CM, Goletz S, Kamaguchi M, Ludwig RJ, Schneider SW, et al. Immunoglobulin M pemphigoid. J Am Acad Dermatol 2021; 85: 1486–1492.
- Fine JD, Neises GR, Katz SI. Immunofluorescence and immunoelectron microscopic studies in cicatricial pemphigoid. J Invest Dermatol 1984; 82: 39–43.
- Arduino PG, Broccoletti R, Carbone M, Conrotto D, Pettigiani E, Giacometti S, et al. Describing the gingival involvement in a sample of 182 Italian predominantly oral mucous membrane pemphigoid patients: a retrospective series. Med Oral Patol Oral Cir Bucal 2017; 22: e149–e152.
- Alexandre M, Brette MD, Pascal F, Tsianakas P, Fraitag S, Doan S, et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore) 2006; 85: 239–252.
- Chan LS, Yancey KB, Hammerberg C, Soong HK, Regezi JA, Johnson K, et al. Immune-mediated subepithelial blistering diseases of mucous membranes. Pure ocular cicatricial pemphigoid is a unique clinical and immunopathological entity distinct from bullous pemphigoid and other subsets identified by antigenic specificity of autoantibodies. Arch Dermatol 1993; 129: 448–455.
- Hecht E, Pitz S, Renieri G. [In-vivo confocal microscopy for the diagnosis of mucous membrane pemphigoid]. Klin Monbl Augenheilkd 2015; 232: 1077–1081.
- Cozzani E, Di Zenzo G, Calabresi V, Carrozzo M, Burlando M, Longanesi L, et al. Autoantibody profile of a cohort of 78 Italian patients with mucous membrane pemphigoid: correlation between reactivity profile and clinical involvement. Acta Derm Venereol 2016; 96: 768–773.
- Dart JK. The 2016 Bowman Lecture Conjunctival curses: scarring conjunctivitis 30 years on. Eye (Lond) 2017; 31: 301–332.
- Grootenboer-Mignot S, Descamps V, Picard-Dahan C, Nicaise-Roland P, Prost-Squarcioni C, Leroux-Villet C, et al. Place of human amniotic membrane immunoblotting in the diagnosis of autoimmune bullous dermatoses. Br J Dermatol 2010; 162: 743–750.
- Maglie R, Borgi A, Caproni M, Antiga E. Indirect immunofluorescence in mucous membrane pemphigoid: which substrate should be used? Br J Dermatol 2019; 180: 1266–1267.
- Hayakawa T, Furumura M, Fukano H, Li X, Ishii N, Hamada T, et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 483–496.
- Jindal A, Rao R, Bhogal BS. Advanced diagnostic techniques in autoimmune bullous diseases. Indian J Dermatol 2017; 62: 268–278.
- Setterfield J, Shirlaw PJ, Kerr-Muir M, Neill S, Bhogal BS, Morgan P, et al. Mucous membrane pemphigoid: a dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol 1998; 138: 602–610.
- Carey B, Joshi S, Abdelghani A, Mee J, Andiappan M, Setterfield J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol 2020; 182: 747–753.
- Xu HH, Werth VP, Parisi E, Sollecito TP. Mucous membrane pemphigoid. Dent Clin North Am 2013; 57: 611–630.