SHORT COMMUNICATION
Morphology of Vessels in Basal Cell Carcinoma in Optical Super-high Magnification Dermoscopy
Joanna POGORZELSKA-DYRBUŚ1, Aimilios LALLAS2 and Jacek C. SZEPIETOWSKI3
1”Estevita” Specialist Medical Practice, Tychy, Poland, 2First Department of Dermatology, Aristotle University, Thessaloniki, Greece and 3Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, Chalubinskiego 1, PL-50-368 Wroclaw, Poland. E-mail: jacek.szepietowski@umw.edu.pl
Citation: Acta Derm Venereol 2023; 103: adv11966. DOI https://doi.org/10.2340/actadv.v103.11966.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 31, 2023; Published: Aug 2, 2023
INTRODUCTION
The prevalence of basal cell carcinoma (BCC), the most common skin cancer, has increased greatly over recent years (1). Diagnosis of BCC is based on clinical examination supported by dermoscopic examination, which increases the accuracy from 60% to over 90% (2, 3). The dermoscopic pattern of BCC has been investigated extensively in several studies. Optical super-high magnification dermoscopy (OSHMD) offers magnification up to 400 ×, enabling the visualization of structures that are invisible or hardly visible in standard 20× magnification (4–6). However, the morphology of the vascular structures of BCC in OSHMD has not been described to date. The aim of this study is to report the morphology of the vessels of BCC in OSHMD.
METHODS
This prospective observational study was conducted in the outpatient clinic. Consecutive cases of BCC, evaluated using standard digital dermoscopy (20× magnification) and OSHMD (400 × magnification), were included in the analysis.
All images were captured using the Medicam 1000 (Fotofinder System, Bad Birnbach, Germany), and OSHMD was conducted with D Scope III lens (Fotofinder System). Medicam 1000 has been used for normal demoscopy, and D-Scope III lens for OSHMD. Minimal pressure was applied, in order to preserve the morphology of the vessels, and ultrasound gel was used as an immersion fluid. The lesions were evaluated for the presence of the following vascular structures: arborizing vessels (linear ramified), short fine telangiectasias (SFT), looped vessels, linear vessels, comma vessels and dotted vessels. The presence of pigmented structures was also recorded as a single variable.
The categorical variables were presented in both absolute numbers and percentages, while continuous variables after assessment for normality with the use of Shapiro-Wilk test were presented as median (quartile 1 – quartile 3). Between-group comparisons were conducted with the use of Pearson’s χ2 test. The interval of 2-sided p < 0.05 was considered statistically significant. STATISTICA 10 (StarSoft Inc., Tulsa, OK, USA) was used for all calculations.
RESULTS
A total of 41 consecutive BCCs, histopathologically classified as nodular (61.0%), superficial (26.8%), multifocal (9.8%) or infiltrative (2.4%), were analysed. The study population comprised 24 women (58.5%) and 17 men (41.5%) with a median age of 67 years. Most of the lesions were located on the trunk (48.8%) and the face (43.9%).
The analytic results of the evaluation with conventional dermoscopy and OSHMD are shown in Table I. In OSHMD, looped vessels were seen in 63.4% (Fig. 1A, B) and arborizing vessels in 53.7% of BCCs (Fig. 1C, D). In a considerable percentage of tumours (53.7%), a previously undescribed type of vessels was also observed in OSHMD, namely thin linear vessels circumferential to pigmented structures (Fig. 1E, F).
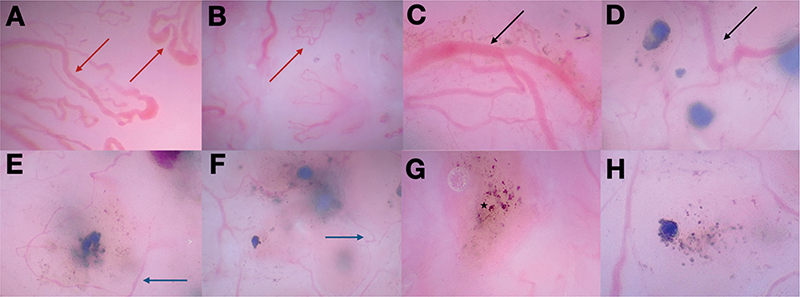
Fig. 1. (A–B) Loop vessels and (C–D) arborizing tree-like vessels visible in optical super-high magnification dermoscopy (OSHMD) indicated with, respectively, red arrows and black arrows. (E–F) Thin linear vessels indicated with blue arrows and (G–H) areas of pigmentation. An asterisk in (G) indicates a melanophage. All images were taken with 400x magnification.
The percentage of looped vessels was significantly higher in OSHMD than in standard dermoscopy (63.4% vs 29.2%, p = 0.02). Arborizing vessels were seen at the same frequency in both magnifications (53.7%, p = 1.00). Pigmented structures were more common in OSHMD images than in standard dermoscopy (56.1% vs 34.1%, p = 0.045), with the identification of individual cells, including melanophages (Fig. 1G, H).
DISCUSSION
This study investigated the vascular pattern of BCC under OSHMD. The results showed that vessels are very frequent in BCC, and the predominating types are looped and arborizing vessels, as well as thin linear vessels circumferential to pigmented structures.
The high frequency of vessels in BCC is very well known from numerous studies using conventional dermoscopy. Considering that OSHMD uses much higher magnifications, that enhance the visualization of vascular structures, the detection of vessels in every single BCC, reflecting the increased neoangiogenesis, was not surprising (7, 8). However, analysis of the morphological type of the vascular structures revealed some novel findings and significant differences between conventional dermoscopy and OSHMD. The modified visualization of features at different magnifications has been shown previously in a study that analysed 400 BCCs with dermoscopy at slightly higher magnifications (50 × and 70 ×) (9).
The most frequent type of vessels in OSHMD of BCC was looped vessels (63.4%), which are infrequently seen with conventional dermoscopy (10–12). This can probably be explained by the fact that these vascular loops are hardly visible at low magnifications. The second most frequent type was arborizing vessels, which are considered the dermoscopic hallmark of BCC. In OSHMD, they project as prominent stem vessels giving rise to numerous branches.
In OSHMD, an additional type of vascular structure was also observed, namely thin linear vessels circumferential to pigmented structures. These vessels may reflect an increased microvascular density surrounding and supporting nests of basaloid cells.
Other types of analysed vessels, namely SFT, dotted and comma vessels were visible only in standard dermo-scopy with frequencies comparable to those previously reported in the literature, while in OSHMD these structures were not reported at all. A likely explanation is that higher magnifications reveal loops and convolutions that are invisible with conventional dermoscopy.
Although not included in the study aims, in OSHMD pigmented structures were also frequently observed, including fine aggregates of cells with pigment that possibly correspond to initial stage of dots or globules (13). Moreover, single melanophages were also visible. However, the diagnostic significance of these tiny, pigmented structures, unrecognizable in standard dermoscopy, is unknown.
This study has a number of imitations. First, the study was performed in a small number of cases. Secondly, the assessment of structures in standard dermoscopy and OSHMD has been performed simultaneously, without crossover blinding. Thirdly, the study did not include a control group and, therefore, the value of the reported findings to discriminate BCC from other tumours cannot be assessed.
This study demonstrates that the use of OSHMD adds novel insights to the submacroscopic morphology of BCC. As research into OSHMD is only preliminary, further studies are required to investigate the patterns of BCC and other common benign and malignant tumours and to assess the potential value of the method in clinical practice.
REFERENCES
- Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol 2017; 177: 359–372.
- Reiter O, Mimouni I, Gdalevich M, Marghoob AA, Levi A, Hodak E, Leshem YA. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol 2019; 80: 1380–1388.
- Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy, WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol 2000; 136: 1012–1016.
- Dusi D, Rossi R, Simonacci M, Ferrara G. Image gallery: the new age of dermoscopy: optical super-high magnification. Br J Dermatol 2018; 178: e330.
- Pogorzelska-Dyrbuś J, Szepietowski JC. Optical super-high magnification dermoscopy of pigmented and nonpigmented nodular basal cell carcinoma. J Cosmet Dermatol 2022; 21: 6458–6460.
- Cinotti E, Rossi R, Ferrara G, Tognetti L, Rubegni P, Perrot JL. Image gallery: super-high magnification dermoscopy can identify pigmented cells: correlation with reflectance confocal microscopy. Br J Dermatol 2019; 181: e1.
- Guerrero-Putz MD, Santana-Gutierrez A, Kubelis-López DE, Villarreal-Martínez A, Chavez-Alvarez S, Salerni G, et al. Uncoiled linear looped vessels in basal cell carcinoma: a novel morphology. Clin Exp Dermatol 2022; 47: 976–978.
- Pogorzelska-Dyrbuś J. “Oak-leaf-like” loop vessels in super-high magnification dermoscopy of basal cell carcinoma. Dermatol Pract Concept 2022; 12: e2022147.
- Seidenari S, Bellucci C, Bassoli S, Arginelli F, Magnoni C, Ponti G. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol 2014; 94: 677–682.
- Lupu M, Caruntu C, Popa MI, Voiculescu VM, Zurac S, Boda D. Vascular patterns in basal cell carcinoma: dermoscopic, confocal and histopathological perspectives. Oncol Lett 2019; 17: 4112–4125.
- Micantonio T, Gulia A, Altobelli E, Di Cesare A, Fidanza R, Riitano A, et al. Vascular patterns in basal cell carcinoma. J Eur Acad Dermatol Venereol 2011; 25: 358–361.
- Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol 2010; 63: 377–388.
- Lallas A, Apalla Z, Argenziano G, Longo C, Moscarella E, Specchio F, Raucci M, Zalaudek I. The dermatoscopic universe of basal cell carcinoma. Dermatol Pract Concept 2014; 4: 11–24.