SHORT COMMUNICATION
Everolimus-induced Symmetrical Drug-related Intertriginous and Flexural Exanthema
Emily Y. KIM1, Katherine C. AWH1,2 and Cecilia A. LAROCCA1,2
1Center for Cutaneous Oncology, Dana-Farber Brigham Cancer Center, Boston, MA and 2Department of Dermatology, Brigham and Women’s Hospital, 41 Louis Pasteur Avenue, Boston MA 02115, USA. E-mail: clarocca@bwh.harvard.edu
Citation: Acta Derm Venereol 2023; 103: adv12197. DOI: https://doi.org/10.2340/actadv.v103.12197.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 30, 2023; Published: Dec 19, 2023
INTRODUCTION
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), first coined as “baboon syndrome”, is a skin eruption with characteristic symmetrical erythematous papules and/or plaques involving the gluteal and intertriginous areas after exposure to systemic drugs (1, 2). Notably, patients lack systemic symptoms. We describe here a case of SDRIFE that developed after initiation of everolimus for neuroendocrine cancer of the pancreas. Everolimus is an oral protein kinase inhibitor of mammalian target of rapamycin (mTOR) that is used as an immunosuppressant therapy for solid organ transplantation or as treatment for various cancers. It is US Food and Drug Administration (FDA) approved as Afinitor® to treat patients with progressive pancreatic neuroendocrine tumours (pNET) that cannot be removed by surgery or that have metastasized (3).
CASE REPORT
The patient is a 64-year-old male with well-differentiated carcinoid neuroendocrine tumour of the pancreatic tail with metastasis to the liver. He was evaluated for a new skin eruption that developed 3 days after starting everolimus 10 mg daily. He first noticed a pink, itchy, bumpy rash in his axilla that quickly spread to involve his groin, behind the knees, and buttocks. He took everolimus for a total of 5 days before stopping it.
Other medications he was taking included aspirin, atorvastatin, insulin glargine, lorazepam, metformin, octreotide, and occasional ibuprofen and cannabidiol (CBD). He was taking these medications consistently for over 4 years. The patient’s last contrast exposures were an abdominal magnetic resonance imaging (MRI) with gadobutrol contrast 3 weeks previously, and a computed tomography (CT) scan of the chest, abdomen and pelvis with iohexol contrast 6 months prior to onset of the rash. Previously the patient had undergone at least 13 MRI scans and 12 CT scans between pancreatic cancer diagnosis and onset of the skin lesions.
The patient overall felt well and denied fever, chills, or malaise. Physical examination showed numerous pink oedematous papules coalescing into plaques in the bilateral axilla and surrounding axillary vault, buttock including the gluteal cleft, and superior posterior thigh (Fig. 1). There were numerous pink coalescing papules in the bilateral popliteal fossae and bilateral inguinal folds, without genital involvement. Everolimus was discontinued and the patient was treated with topical betamethasone cream and a short prednisone taper, and the rash resolved after 2 days. He had no recurrence of the skin lesions with further MRI or CT scans or continuation of his other medications. His complete blood count with differential and complete metabolic panel 1 week after discontinuing everolimus were relatively unchanged compared with prior to starting the medication, except for slightly elevated absolute lymphocyte and monocyte counts, at 3.71 and 1.00 K/uL, respectively.
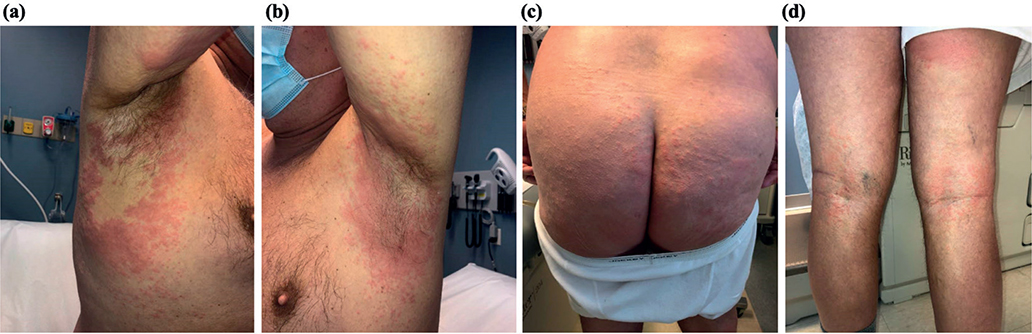
Fig. 1. Erythema and oedema on: (a and b) bilateral axilla, (c) buttocks, and (d) bilateral popliteal fossae.
DISCUSSION
SDRIFE was originally described in 1984 as baboon syndrome due to the characteristic well-demarcated bright-red eruption on the buttocks and genital area, similar to the bottom of a baboon (4). It is caused by exposure to systemic drugs, most often antibiotics, such as amoxicillin, trimethoprim/sulfamethoxazole, pristinamycin, clindamycin, erythromycin, and metronidazole. In addition, antimicrobial drugs, such as terbinafine, nystatin, fluconazole, and valacyclovir, have also been reported as causative factors (1, 5). Other reported triggers of SDRIFE are, but are not limited to, anti-asthmatics, allopurinol and monoclonal antibodies. In the current case, SDRIFE was attributed to everolimus given the short temporal association between its initiation and the onset of the patient’s skin eruption (3 days). The current patient lacked any systemic symptoms and had been taking his other medications for several years. While a controlled drug-provocation test can be useful for diagnosis, out-comes of the test can be highly variable (1). Because there were several other therapeutic options for the patient’s pancreatic cancer, as well as considerable evidence for everolimus as the culprit drug, rechallenge was not pursued. In addition, the patient received further MRI and CT scans without incident. In the literature, there is 1 previous report of everolimus-induced SDRIFE (6).
While SDRIFE can occur just a few days after initiation of a drug, onset can also occur from a few hours to a few days after removal of the causative drug (1, 2). It is suspected to develop as a result of a type IV delayed hypersensitivity immune response; however, this does not explain the occurrence of SDRIFE after the first exposure to a drug without prior sensitization (7). Diagnosis relies mostly on clinical presentation and history, and exclusion of other causes of flexural eruptions (e.g. inverse psoriasis, allergic contact dermatitis, scabies) (2). Laboratory investigations may be performed to exclude systemic involvement but are not necessary for diagnosis of SDRIFE.
In conclusion, we present here a case of SDRIFE most likely caused by everolimus, which has only been previously reported once in the literature. Dermatologists as well as physicians who prescribe systemic drugs should be aware of this drug eruption if a patient develops symmetrical erythematous papules and/or plaques on the gluteal and intertriginous areas, but lacks systemic symptoms. While the presentation can be alarming, SDRIFE can be managed with topical and oral glucocorticoids, and patients may even resume the culprit drug once the eruption clears, if it is needed.
REFERENCES
- Harbaoui S, Litaiem N. Symmetrical drug-related intertriginous and flexural exanthema. StatPearls Publishing; 2021 [cited 2022 Sep 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539750/
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 2004; 51: 297–310.
- Hasskarl J. Everolimus. In: Martens UM, editor. Small Molecules in Oncology [Internet]. Cham: Springer International Publishing; 2018 [cited 2022 Sep 9]. p. 101–23. (Recent Results in Cancer Research). Available from: https://doi.org/10.1007/978-3-319-91442-8_8
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis 1984; 10: 97–100.
- Schuler AM, Smith EH, Chaudet KM, Bresler SC, Gudjonsson JE, Kroshinsky D, et al. Symmetric drug-related intertriginous and flexural exanthema: clinicopathologic study of 19 cases and review of literature. J Cutan Pathol 2021; 48: 1471–1479.
- Kurtzman DJB, Oulton J, Erickson C, Curiel-Lewandrowski C. Everolimus-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE). Dermat Contact Atopic Occup Drug 2016; 27: 76–77.
- Tan SC, Tan JWL. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol 2011; 11: 313–318.