ORIGINAL REPORT
Clinical Approach to Patients with Moderate-to-Severe Atopic Dermatitis: A Spanish Delphi Consensus
Jose J. PEREYRA-RODRIGUEZ1, Esther S. BALDRICH2, Ricardo RUIZ-VILLAVERDE3, Eulalia B. TORRES4, Pablo DE LA C. DOBAO5, Ignasi F. NART6, Ángeles F. MENÉNDEZ7, Ana MARTIN-SANTIAGO8, Javier M. MIQUEL9, Juan F. SILVESTRE10 and Jose C. ARMARIO-HITA11
Departments of Dermatology, 1School of Medicine, University of Seville, 2Santa Creu i Sant Pau Hospital, Barcelona, 3Clínico San Cecilio University Hospital, Granada, 4Sant Joan de Déu Hospital, Barcelona, 5Infanta Leonor University Hospital, Madrid, 6Bellvitge University Hospital, Barcelona, 7Pontevedra University Hospital, Pontevedra, 8Son Espases University Hospital, Palma de Mallorca, 9Arnau de Vilanova Hospital, Valencia, 10Department of Dermatology, Alicante University General Hospital, Alicante and 11Department of Dermatology, Puerto Real University Hospital, Cádiz, Spain
Despite emerging evidence and advances in the management of atopic dermatitis there a lack of consensus regarding the diagnostic criteria, therapeutic approach, method to assess severity, and patient follow-up for this condition. An expert consensus study was conducted to provide recommendations on the management of patients with moderate-to-severe atopic dermatitis. The study used Delphi-like methodology based on a literature review, a summary of the scientific evidence, and a 2-round survey. The agreement of 60 panellists on 21 statements was evaluated. Consensus was predefined as ≥ 80% agreement of all respondents. In the first round 6 statements reached consensus. Unanimous consensus was achieved regarding therapeutic goals and patient satisfaction (maintained in the long term and periodic goals reassessment recommended every 3–6 months). In the second round, half of the statements reached consensus, all related to patient follow-up, treatment goals, and atopic comorbidities. The statements that did not reach consensus were related to diagnosis (biomarkers, allergy, and food testing) and starting patients on conventional systemic treatment rather than advanced treatment. The study assessed expert opinion regarding a variety of topics related to the clinical approach to patients with moderate-to-severe atopic dermatitis, in order to provide guidance on the diagnosis and management of patients with atopic dermatitis.
Key words: atopic dermatitis; immunoglobulin E; biological agents; type 2 inflammatory diseases.
SIGNIFICANCE
Atopic dermatitis is a chronic disease that causes inflammation, redness and irritation of the skin. Despite numerous advances in the management of this disease there is a lack of consensus on the diagnostic criteria and treatment approach. This Spanish expert consensus study provides recommendations on the management of patients with moderate-to-severe atopic dermatitis, in order to standardize the approach and guide health professionals in diagnostic and therapeutic decision-making.
Citation: Acta Derm Venereol 2023; 103: adv12314. DOI https://doi.org/10.2340/actadv.v103.12314.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 12, 2023; Published: Nov 20, 2023
Corr: Esther Serra Baldrich, Dermatology Department, Hospital de la Santa Creu i Sant Pau, Sant Antoni Maria Claret, 167, ES-08025 Barcelona, Spain. E-mail: eserra@santpau.cat
Competing interests and funding: JJP-R has received honoraria for research from Novartis, Abbvie, Galderma, Leo-Pharma, Lilly, and Sanofi, and for lecturing and other financial benefit from Abbvie, Almirall, Galderma, Janssen, Gebro-Pharma, Leo-Pharma Novartis, Lilly, Novartis, Pfizer, Sanofi and UCB. ES has received funding for participating in research, lecturing and consultancy from Novartis, Almirall, Genentec, Pierre Fabre, Pfizer, Abbvie, Lilly, Leo Pharma, Sanofi, Galderma. EB has received funding for attendance at meetings and conferences from Pierre-Fabre dermatology, Novartis and Sanofi; speaker fees from AbbVie, Sanofi, Leti, Isdin, LeoPharma; and funding for participating in research from AbbVie, Lilly, Leo Pharma, Novartis, Pfizer, Galderma, Regeneron and Sanofi. PdlC has been a consultant and advisor and/or received speaking fees and/or grants and/or served as an investigator in clinical trials for the following companies: Abbvie, Almirall, Astellas, Biogen, Boehringer, Celgene, Janssen., LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB. AF has received funding for attendance at meetings and conferences, speaker fees and funding for participating in research from Sanofi, Almirall, Abbvie, Leo Pharma, Janssen, Novartis, Amgen, UCB Pharma, Lilly and Pfizer. JFS has served as a consultant and received speaking fees at educational events for Sanofi, Regeneron, Abbvie, Eli Lilly, Galderma, LEOPharma, Novartis, Pfizer, and has served as the principal investigator in clinical trials sponsored by AbbVie, Amgen, Bristol Meyer Squibb, Eli-Lilly, Incyte, Leo Pharma, Novartis, Pfizer and Sanofi. AM-S has received funding for attendance at meetings and conferences from AbbVie, Pierre-Fabre, Sanofi, UCB Pharma; speaker fees from Leo Pharma, Leti, Lilly, Novartis, Sanofi; and funding for participating in research from AbbVie, Pfizer and Sanofi. RR-V has received funding for attendance at meetings and conferences, speaker fees and funding for participating in research from Sanofi, Abbvie, Lilly, Novartis, Galderma, Pfizer, UCB Pharma, Janssen. JM has received funding for attendance at meetings and conferences, speaker fees and funding for participating in research from Sanofi, Abbvie, Leo Pharma, Janssen, Novartis, Amgen, UCB Pharma and Leo Pharma. IF has received funding for attendance at meetings and conferences, and speaker fees from Sanofi, Abbvie, Lilly, Leo Pharma, Novartis, Pierre Fabre, La Roche Posay. JCA-H has received funding for attendance at meetings and conferences, speaker fees and funding for participating in research from Sanofi, Abbvie, Lilly, Novartis, Galderma, Pfizer, UCB Pharma, Janssen.
INTRODUCTION
A topic dermatitis (AD) is a systemic chronic, pruritic, inflammatory skin disease with a relapsing course that occurs both in children and adults (1, 2). AD typically starts in childhood, affecting approximately 15–20% of children worldwide and 1–3% of the adult population (3, 4), with large variations among countries and regions. In Spain, the prevalence in adults is approximately 55.7% for mild AD, 38.2% for moderate AD, and 6.1% for severe AD (5).
In childhood, patients with AD can develop a broad spectrum of allergic comorbidities, such as allergic rhinitis, asthma, food allergies or eosinophilic esophagitis (1, 6, 7). The relationship between these conditions and AD is well established and type 2 inflammation can explain an underlying mechanism for the development of inflammation and barrier defects (8, 9). Interleukins (IL)4 and IL13 have a central role in type 2 inflammation, facilitate the production of immunoglobulin E (IgE), and are associated with barrier dysfunction (10, 11).
The cardinal features of AD are dry skin and severe pruritus. However, clinical manifestations depend on age, ethnicity, and stage of the disease and can range from papulovesicles to lichenified plaques (12). AD is not a life-threatening condition, but it poses a significant social, psychosocial, and economic burden. The impact of AD on quality of life is well established, with itching, scratching, sleep loss, and social embarrassment being among the most frequently difficulties (13).
There is no definitive test for diagnosis of AD. It is diagnosed clinically based on historical features, morphology and distribution of skin lesions, and associated clinical signs (2, 12). Several sets of criteria have been proposed, some of which are poorly defined or non-specific, while others are quite specific but uncommon (2); therefore, there are no standardized diagnostic criteria in clinical practice.
Moreover, it is difficult to establish a universal method to assess the severity of AD, due to its heterogeneous course (14). Markers that may reflect disease severity or activity include blood eosinophil levels, serum IgE, lactate dehydrogenase (LDH), or thymus activity-regulating chemokine (TARC) (15, 16). Severity indices have also been developed, such as Severity Scoring of Atopic Dermatitis (SCORAD) or Eczema Area and Severity Index (EASI). Quality of life questionnaires and indices to assess long-term disease control, such as the Atopic Dermatitis Control Tool (ADCT), are also used (2, 17).
Topical treatments are the mainstay of AD therapy, but systemic treatments are often required in combination (18). Although AD is considered to involve multiple immune pathways, the activation of type 2 immune responses, driven by innate type 2 lymphoid cells and T helper 2 (Th2) cells, and type-2 cytokines (mainly IL-4 and IL-13), appears to be a dominant mechanism (18). Taking this mechanism into consideration, agents that can inhibit 1 or more cytokines, block growth factors and hormone receptor signalling pathways, such as dupilumab, tralokinumab or Janus kinase (JAK) inhibitors, have been developed recently. These therapies constitute the current treatment paradigm (18).
Despite emerging evidence and numerous advances in the management of AD, there is a lack of consensus on the most appropriate criteria for diagnosis, as well as the optimal therapeutic approach, specifically for transition from conventional to advanced therapies. There is uncertainty surrounding the method to assess severity or patient follow-up. Due to the lack of clear consensus, this study aimed to provide recommendations on the management of patients with moderate-to-severe AD through consultation with a panel of experts, using a Delphi-like methodology. These expert recommendations aim to unify the approach to the management of these patients and guide diagnostic and therapeutic decision-making.
MATERIALS AND METHODS
This consensus study is based on a literature review, a summary of the available scientific evidence, and the use of a 2-round Delphi-type consensus survey. The Delphi method is a structured process designed to systematically collect expert’s opinion on a given subject (19).
A scientific committee was formed, consisting of 11 Spanish experts from a representative sample of university hospitals throughout Spain with significant experience in the management of patients with moderate-to-severe AD, 2 of whom have expertise in paediatric patients. The committee members are leaders in AD and treat most of the cases of moderate-severe AD in their area (an estimated mean of 150 patients/year). The scientific committee was responsible for the decision-making, developed the Delphi statements based on the results of the systematic review, and adapted them before the second round.
A research protocol was developed that described the objectives and methodology of the project, and the criteria and requirements for the selection of survey respondents. The scientific committee developed 18 clinical questions (Table I), following the PICO format (Patient, Intervention, Comparison, Outcomes) (20).
In October 2021, a rapid systematic review was conducted of the following clinical databases: National Guideline Clearinghouse, National Library of Guidelines, TRIP database, Epistemonikos, and Medline. Clinical practice guidelines, meta-analyses, systematic reviews, randomized clinical trials, and observational studies, and only publications in English or Spanish from the last 5 years were reviewed. If no data were found in these documents, the search was repeated without a time filter.
Overall, 2,273 publications were identified, of which 244 were duplicates. The title and abstract of 2,030 references were evaluated according to the previously established inclusion and exclusion criteria, and 87 references were selected for a full reading. Subsequently, 26 were discarded because they did not meet the inclusion criteria, and 61 publications were finally included in the evidence synthesis. The searches and study selection processes are detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram shown in Fig. 1.
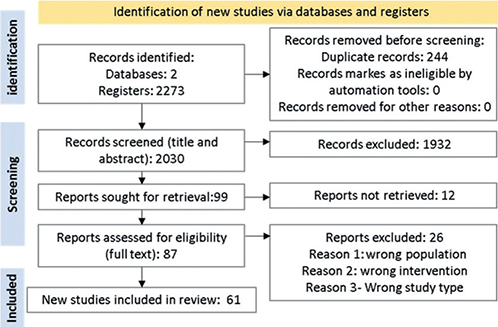
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2021 flow diagram of the databases and registry search performed for the systematic literature review.
The complete text of the selected articles was read critically and the information was synthesized. The studies were classified according to the quality of the work using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE). Certainty of evidence recommendation strengths are detailed in Table II.
Based on the evidence synthesis, a draft was developed to answer the 18 clinical questions. The scientific committee revised the draft and agreed on 21 statements to be included in the Delphi-type questionnaire (Table II).
A panel of 60 participants was selected from a representative series of hospitals and geographical areas. Panellists had to meet the following criteria: (i) specialists in dermatology, (ii) experienced in the management of patients with AD; (iii) members of scientific society working groups; (iv) seeing a minimum of 10 patients with AD per month.
The consensus process was conducted using the Delphi 2-round methodology. The questionnaire was made available on an online platform that offered access to the summary of the evidence.
The degree of agreement was assessed on a scale of 1–5 (1: strongly disagree, 2: moderately disagree, 3: neither agree nor disagree, 4: moderately agree, 5: strongly agree). Consensus was predefined as ≥ 80% of all respondents rating their agreement as 4 or 5, and unanimous consensus was defined as 100% agreement (all voting 5). After the first round, the results and comments of the panellists were analysed, the relevant modifications were made to the statements, and submitted to the second round. Statements that reached consensus in the first round were not submitted to the second round.
RESULTS
All 60 invited panellists participated in the first round and 58 took part in the second round (96% participation). Of all the panellists, 94.8% worked in a hospital setting, and 84% of them had more than 10 years of experience. In total, almost 90% of panellists saw between 10 and 50 patients with AD per month.
In the first round, 21 Delphi statements were evaluated and 6 reached consensus, 2 of them unanimous. Fifteen other statements were reviewed and re-submitted in the second round. To improve clarity and facilitate the consensus, some of the statements were divided into 2 statements. In the second round, 9 statements achieved consensus and 9 were discrepant. The flow of the 2 Delphi rounds and its results (% of agreement and round) are shown in Fig. 2 and Table II, respectively.
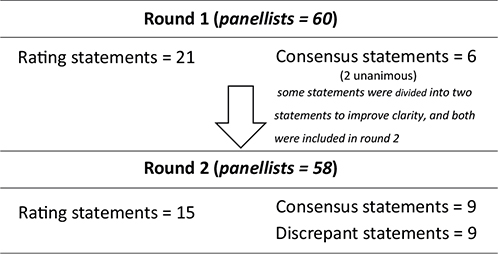
Fig. 2. Flow diagram illustrating the 2 rounds of the Delphi process.
Out of the 8 statements from section 1 (moderate-to-severe AD diagnosis), 1 item achieved unanimous agreement, consensus was reached for 2 items, whereas divergence was observed in 5 items. It must be noted that 1 of these statements was modified after the first round and 2 of its versions were validated in the second round. In the treatment section (Section 2. Moderate-to-severe AD treatment), 6 statements achieved agreement and 4 were still disagreed upon after the second round. Consensus was reached on 2 statements in section 3 (AD patient’s follow-up) in the first round, with 1 of the statements achieving unanimous agreement. Agreement was reached on the remaining 4 statements after the second round. Consensus was reached on the statements in section 4 about atopic comorbidities in the first round.
DISCUSSION
This Delphi-based study focused on unresolved issues on the optimal strategy for the therapeutic management of patients with moderate-to-severe AD. A 2-round Delphi consensus explored the opinions of experienced dermatologists across Spain regarding a series of statements made after a systematic review.
The results of this study show a unanimous agreement regarding the use of EASI, SCORAD, and Investigator Global Assessment (IGA) to assess disease severity. Although there is a lack of uniformity in disease-severity scales, all the panellists approve the use of the 3 aforementioned scales, as it is suggested in the available literature (2, 21). In clinical practice, SCORAD followed by IGA and EASI are the most used scales to assess disease severity (21). Regarding the use of biomarkers for clinical and severity assessment, panellists considered that neither IgE nor LDH can be recommended. These results are aligned with current evidence (22). Although IgE is 1 of the most studied biomarkers, correlation with disease severity is quite weak and LDH could prove useful, but it requires additional research (22). To date, TARC levels are the most reliable biomarker studied with the best correlation with disease severity, but they are not routinely available in most laboratories (15, 22).
The role of allergy in AD is controversial. AD itself is not a type I or type IV allergy, nor is it necessarily associated with allergic sensitization. However, overall, the data indicates that allergy plays a role in certain patients (23). In this sense, agreement was reached by considering allergy testing (patch tests for delayed-type IV hypersensitivity) in patients with AD, specifically in those with a history of contact dermatitis, atypical lesion distribution, or adulthood onset. As evidenced by the literature, allergy testing is recommended when allergic contact dermatitis is suspected (24). Panellists, however, did not show agreement on performing a skin-prick test (SPT) or a serum-specific IgE testing to food and inhaled allergens for the diagnosis.
Several studies have shown an increased rate of sensitization to both food and aeroallergens (25). Although these proportions vary widely, on average, 50% of children and 35% of adults with AD are sensitized to common allergens (26, 27). Patients with AD commonly have elevated total IgE that can lead to non-specific IgE binding on allergen-specific IgE assays, raising the risk of false-positive results (23). Considering this information, the panellists do not agree that a SPT for food or aeroallergen-specific IgE testing should be carried out in the diagnostic evaluation of patients with AD. These tests should only be performed when the patient has a compatible clinical history of allergy. Panellists also did not agree with routinely prescribing an elimination diet in patients who have undergone allergy testing and showed positive SPT or serum-specific IgE to food allergens without clinical correlation. Despite the fact that elimination of food allergens in selected patients with AD and confirmed food allergy can lead to significant clinical improvement (28, 29), foods should not be eliminated from the diet randomly without firm clinical suspicion, especially since, in general, foods have a low rate of triggering AD (30). Test results should always be correlated with clinical history and clinical reactivity (23).
Topical treatments are the cornerstone of AD therapy (18). Consensus was reached for the proactive use of corticosteroids or calcineurin inhibitors for the treatment of moderate-to-severe AD. The results of the current study are similar to those reported by another group of Spanish dermatologists (31) and are aligned with current evidence (32, 33). Although the percentage of agreement was very high (almost 78%), our panellists did not agree with prioritizing topical calcineurin inhibitors over topical corticosteroids. Lack of consensus may be due to the limited evidence comparing both therapeutic groups and safety alerts related to calcineurin inhibitors (33, 34). Narrowband ultraviolet B (NBUVB) phototherapy compared wtih placebo improved eczema severity (including itching), and it is considered an alternative first-line therapy for adults and adolescents with moderate-to-severe AD (35). Considering current evidence, it is surprising that panellists did not agree with the use of phototherapy. It is believed that the wording of this item could have been misinterpreted, or that the lack of agreement could be related to the unavailability of photo-therapy or the fact that the procedure is time- consuming. Consensus was reached for the use of Patient Reported Outcomes (PRO), such as numerical rating scale (NRS) pruritus, and quality of life scales, in addition to objectives scales to assess disease severity.
Patients with moderate-to-severe AD despite optimal topical therapy will require a systemic treatment for adequate disease control (36). The choice of treatment is based on evidence of efficacy, safety, availability, patient preference, and cost considerations. Statements 13 and 14 were expert opinion statements based on current prescription regulations from the Spanish national health system, not based on evidence, and the use of 1 conventional systemic treatment before starting an advanced systemic treatment (dupilumab, tralokinumab or oral JAK inhibitors, such as abrocitinib, baricitinib, filgotinib, tofacitinib and upadacitinib) is recommended both in elderly patients and in childhood.
Panellists agreed to initiate treatment with an advanced systemic treatment (biologics and approved oral JAK inhibitors) over systemic conventional treatments for better control of the disease. In line with these results, a recent systematic review about the short-term effectiveness and safety of biologics and JAK inhibitors concluded that upadacitinib and abrocitinib are the drugs with the highest efficacy, both in monotherapy and in combination with topical corticosteroids. However, these drugs were also associated with the highest risk of adverse effects, whilst monoclonal antibodies have a better safety profile (37).
JAK inhibitors are new molecules that partially block multiple cytokine signalling, including IL-4, IL-5, and IL-13, involved in immune response and inflammation. Baricitinib and upadacitinib are JAK inhibitors commercialized in Spain for the treatment of AD. The panel of experts agreed that, in patients refractory to systemic therapy and candidates for advanced treatment with infectious comorbidities and thromboembolic risk, biologic treatments specifically targeting type 2 inflammation are preferred over oral JAK inhibitors due to their safety profile. They also agreed on the use of biologics in children (< 12 years of age) and in elderly patients (> 65 years). In the elderly population, it can be deduced that the preference of the panellists to use a biological treatment over an oral JAK inhibitor is based on the safety profile. These results are consistent with current safety data on the use of JAK inhibitors, and recent recommendations from the European Commission Pharmacovigilance Risk Assessment Committee (PRAC) to minimize the risk of serious side-effects with JAK inhibitors. These side-effects include cardiovascular conditions, blood clots, cancer and serious infections. These medicines should be used in the following patients only if no suitable treatment alternatives are available: those aged ≥ 65 years, those at increased risk of major cardiovascular problems (such as heart attack or stroke), those who smoke or have done so for a long time and those at increased risk of cancer (38). Regarding paediatric patients, it must be considered that in patients < 12 years old JAK inhibitors are not yet approved in Spain and only dupilumab can be used in this population. Hence, experts consider that consensus was reached for this statement due to the lack of indication.
Section 3 of the statements focused on the optimal follow-up of patients with AD. Panellists recommend considering a change in treatment if at least EASI75 or if at least SCORAD 50 are not achieved after 3–6 months of treatment. These results align with the algorithm of an international consensus for decision-making in treating moderate-to-severe AD with systemic treatments (39). SCORAD has been proven to be an appropriate score to detect the progress of AD, and EASI is suitable for monitoring drug efficacy (21). During the clinical assessment of patients, the objective of improving patient satisfaction must be individualized, depending on the predominant signs/symptoms and disease course. These objective and therapeutic goals should be maintained in the long term and a reassessment is recommended every 3–6 months.
As previously stated, patients with AD can develop atopic comorbidities, such as allergic rhinitis, asthma, food allergies, or eosinophilic oesophagitis, referred to as “the atopic march” (1, 6, 7). In patients with moderate-to-severe AD, dupilumab has been shown to significantly improve asthma and/or rhinosinusitis, nasal polyposis and eosinophilic esophagitis compared with placebo (40, 41). Moreover, it has also been demonstrated that it is beneficial for adult patients with perennial allergic conjunctivitis and perennial allergic asthma associated with moderate-to-severe AD (1). In line with this, the experts agreed on the use of dupilumab over other therapies due to its proven effect on clinical improvement, change in asthma control score and sinus symptoms, in addition to its effect on EASI75, NRS, IGA, and Patient Oriented Eczema Measure (POEM) scores.
The current Delphi-like study assessed expert opinion in a variety of topics on the clinical approach to patients with moderate-to-severe AD. The results show that there are fields in disease management with discrepancies, revealing huge clinical variability.
The panel of experts considers that evidence for using biomarkers to assess the severity of moderate-to-severe AD is limited, and none of the biomarkers can be recommended. They also recommend performing allergy testing for type IV hypersensitivity (patch tests), especially when allergic contact dermatitis is suspected by history, there is an atypical distribution of lesions, or in patients with adult-onset AD. There is a broad consensus on the optimal patient follow-up considering EASI75 as a therapeutic goal. Regarding therapeutic options, study results show that advanced systemic treatments (biologics and oral JAK inhibitors) are preferred to conventional systemic treatments if there are no contradictions. Efforts must be made to allow access to advanced systemic treatments as first-line treatment options in patients with moderate-to-severe AD and high burden of disease.
ACKNOWLEDGEMENTS
The authors are grateful to the 60 panellists, specialists in dermatology, who participated in the Delphi panel: Amalia Serrano Gotarredona; Ana María Giménez Arnau; Ánder Zulaica Gárate; Antonio Guilabert; Araceli Sánchez-Gilo; Bibiana Pérez; Cristina de las Heras Sotos; Daniel González Vilas; Enrique Gimeno Carpio; Enrique Gómez de la Fuente; Esther Roe; Fátima Tous Romero; Francisco Javier Ortiz de Frutos; Francisco José Navarro Triviño; Gemma Melé i Ninot; Gloria Garnacho; Hae Jin Suh Oh; Inmaculada Ruiz González; Isabel Belloch; Jorge Spertino; José Manuel Azaña; José Manuel Carrascosa Carrillo; José María Sánchez Motilla; Juan García Gavín; Laia Curto; Leopoldo Borrego Hernando; Manel Velasco Pastor; Marcos Hervella Garcés; María Antonia Pastor Nieto; María Asunción Vicente; María Elena Gatica Ortega; María Rosa Perelló; Maria Teresa Monserrat; Marta Elosua; Marta Feito; Mercedes Rodríguez Serna; Minia Campos; Miquel Armengot Carbó; Mónica Munera-Campos; Pedro Herranz; Pedro Mercader García; Raúl de Lucas; Rebeca Alcalá; Ricardo González Pérez; Ricardo Suárez Fernández; Rosa Mª Fernández Torres; Sabela Rodríguez Blanco; Sara Alcántara-Luna; Susana Córdoba Guijarro; Violeta Zaragoza Ninet; Marc Julià; Rosa Izu; Altea Esteve; Gastón Roustan; Gregorio Carretero; Juan Antonio Ratón; Manuel Galán; Marta García Bustinduy; Yolanda Gilaberte; Javier Domínguez Cruz.
The authors acknowledge Sanofi for economic support for the development of this project and GOC Health Consulting for their methodological and medical writing support.
All the steps of the study were funded by Sanofi. Sanofi did not contribute to the design of the study, the preparation of the questionnaire, or the analysis of the results.
REFERENCES
- Nettis E, Patella V, Lombardo C, Detoraki A, Macchia L, Di Leo E, et al. Efficacy of dupilumab in atopic comorbidities associated with moderate-to-severe adult atopic dermatitis. Allergy 2020; 75: 2653–2661.
- Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015; 66: 8–16.
- Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol 2021; 126: 417–428.e412.
- Sicras-Mainar A, Navarro-Artieda R, Sánchez L, Sastre J. Prevalence of severe atopic dermatitis in adults in 3 areas of Spain. J Investig Allergol Clin Immunol 2018; 28: 195–197.
- Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol 2014; 5: 202.
- Serra-Baldrich E, de Frutos JO, Jáuregui I, Armario-Hita JC, Silvestre JF, Herraez L, et al. Changing perspectives in atopic dermatitis. Allergol Immunopathol (Madr) 2018; 46: 397–412.
- Senna G, Micheletto C, Piacentini G, Schiappoli M, Girolomoni G, Sala G, et al. Multidisciplinary management of type 2 inflammatory diseases. Multidiscip Respir Med 2022; 17: 813.
- Beck LA, Cork MJ, Amagai M, De Benedetto A, Kabashima K, Hamilton JD, et al. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov 2022; 2: 100131.
- Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol 2014; 4: 361–370.
- Humeniuk P, Dubiela P, Hoffmann-Sommergruber K. Dendritic cells and their role in allergy: uptake, proteolytic processing and presentation of allergens. Int J Mol Sci 2017; 18: 1491.
- Weston W, Howe W. Atopic dermatitis (eczema): pathogenesis, clinical manifestations, and diagnosis. 2022. [cited 2022 Oct 5]. Available from: https://www.uptodate.com/
- Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol 2018; 79: 448–456.e430.
- Hong MR, Lei D, Yousaf M, Chavda R, Gabriel S, Janmohamed SR, et al. A real-world study of the longitudinal course of adult atopic dermatitis severity in clinical practice. Ann Allergy Asthma Immunol 2020; 125: 686–692.e3.
- Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, et al. Biomarkers in atopic dermatitisa review on behalf of the International Eczema Council. J Allergy Clin Immunol 2021; 147: 1174–1190.e1.
- Mastraftsi S, Vrioni G, Bakakis M, Nicolaidou E, Rigopoulos D, Stratigos AJ, et al. Atopic dermatitis: striving for reliable biomarkers. J Clin Med 2022; 11: 4639.
- Simpson E, Eckert L, Gadkari A, Mallya UG, Yang M, Nelson L, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol 2019; 19: 15.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360.
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015.
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995; 123: A12–13.
- Iannone M, Tonini G, Janowska A, Dini V, Romanelli M. Definition of treatment goals in terms of clinician-reported disease severity and patient-reported outcomes in moderate-to-severe adult atopic dermatitis: a systematic review. Curr Med Res Opin 2021; 37: 1295–1301.
- Thijs J, Krastev T, Weidinger S, Buckens CF, de Bruin-Weller M, Bruijnzeel-Koomen C, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol 2015; 15: 453–460.
- Spergel JM. Role of allergy in atopic dermatitis (eczema). online] 2022 [cited 2022 Oct 13]. Available from: https://www.uptodate.com/contents/role-of-allergy-in-atopic-dermatitis-eczema?search=atopic%20dermatitis&topicRef=1729&source=see_link#H1.
- Hamann CR, Hamann D, Egeberg A, Johansen JD, Silverberg J, Thyssen JP. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 70–78.
- Schäfer T. The impact of allergy on atopic eczema from data from epidemiological studies. Curr Opin Allergy Clin Immunol 2008; 8: 418–422.
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol 2004; 114: 150–158.
- Eller E, Kjaer HF, Høst A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy 2009; 64: 1023–1029.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev 2008; 2008: Cd005203.
- Uenishi T, Sugiura H, Tanaka T, Uehara M. Role of foods in irregular aggravation of skin lesions in children with atopic dermatitis. J Dermatol 2008; 35: 407–412.
- Lim NR, Lohman ME, Lio PA. The role of elimination diets in atopic dermatitis – a comprehensive review. Pediatr Dermatol 2017; 34: 516–527.
- Sastre J, Baldrich ES, Armario Hita JC, Herráez L, Jáuregui I, Martín-Santiago A, et al. Consensus on the clinical approach to moderate-to-severe atopic dermatitis in Spain: a Delphi survey. Dermatol Res Pract 2020; 2020: 1524293.
- Perälä M, Ahola M, Mikkola T, Pelkonen AS, Remitz A, Mäkelä MJ. Young children with moderate-to-severe atopic dermatitis can be treated safely and effectively with either topical tacrolimus or mild corticosteroids. Acta Paediatr 2020; 109: 550–556.
- Abędź N, Pawliczak R. Efficacy and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis: meta-analysis of randomized clinical trials. Postepy Dermatol Alergol 2019; 36: 752–759.
- Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: a 15-year experience. J Am Acad Dermatol 2016; 75: 410–419.e413.
- Musters AH, Mashayekhi S, Harvey J, Axon E, Lax SJ, Flohr C, et al. Phototherapy for atopic eczema. Cochrane Database Syst Rev 2021; 10: Cd013870.
- Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol 2012; 26: 1176–1193.
- Pereyra-Rodriguez JJ, Alcantara-Luna S, Domínguez-Cruz J, Galán-Gutiérrez M, Ruiz-Villaverde R, Vilar-Palomo S, et al. Short-term effectiveness and safety of biologics and small molecule drugs for moderate to severe atopic dermatitis: a systematic review and network meta-analysis. Life (Basel) 2021; 11: 927.
- European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC). EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. [cited 2022 Dec 20]. Available from https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki.
- De Bruin-Weller M, Biedermann T, Bissonnette R, Deleuran M, Foley P, Girolomoni G, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol 2021; 101: adv00402.
- Boguniewicz M, Beck LA, Sher L, Guttman-Yassky E, Thaçi D, Blauvelt A, et al. Dupilumab improves asthma and sinonasal outcomes in adults with moderate to severe atopic dermatitis. J Allergy Clin Immunol Pract 2021; 9: 1212–1223.e6.
- Hamilton JD, Harel S, Swanson BN, Brian W, Chen Z, Rice MS, et al. Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases. Clin Exp Allergy 2021; 51: 915–931.