SHORT COMMUNICATION
Vellus-to-terminal Hair Follicle Reconversion Occurs in Male Pattern Balding and is Promoted by Minoxidil and Platelet-rich Plasma: In Vivo Evidence from a New Humanized Mouse Model of Androgenetic Alopecia
Amos GILHAR1 , Aviad KEREN1 and Ralf PAUS2–4
, Aviad KEREN1 and Ralf PAUS2–4
1Skin Research Laboratory, Rappaport Faculty of Medicine, Technion – Israel Institute of Technology, Haifa, Israel, 2Dr Phillip Frost Department of Dermatology & Cutaneous Surgery, Miller School of Medicine, University of Miami, Miami, FL, USA, 3Monasterium Laboratory, Münster, Germany and 4CUTANEON, Hamburg, Germany. E-mail: doritg2000@gmail.com
Citation: Acta Derm Venereol 2023; 103: adv12320. DOI https://doi.org/10.2340/actadv.v103.12320.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 15, 2023; Published: Oct 18, 2023
Competing interests and funding: AG, AK and RP state no conflict of interest. For the record, in their laboratories AG and RP perform contract preclinical hair research for competing industry clients, and RP is founder & CEO of Monasterium Laboratory and CUTANEON, Germany.
INTRODUCTION
It has recently been claimed that, once terminal (T) scalp hair follicles (HFs) have been fully miniaturized into vellus (V) HFs in male pattern androgenetic alopecia (mpAGA), these cannot be reconverted into T HFs, even under therapy (1), and that even long-term treatment with topical minoxidil 5% (MXL) or oral finasteride fails to significantly change the number of V HFs (1). Rushton & Van Neste et al. (1) reported that hair regrowth following treatment for AGA is mostly due to an increase of HFs in kenogen, i.e. when the HF does not contain a visible hair shaft (2). Instead, these authors postulated that T HFs in a period of relative dormancy are re-activated by mpAGA therapeutics and that any increase in the number of hair shafts observed post-therapy primarily reflects anagen initiation in kenogen HFs (1).
This provocative concept stands in sharp contrast to prior histological evidence (3), and would have major clinical consequences: it would mandate early intervention in AGA management after which only hair transplantation and, perhaps, selected cell-based therapies would be therapeutically meaningful (4, 5).
Yet, the fact that hypertrichosis-inducing drugs (e.g. MXL, cyclosporine A) and hormones (e.g. androgens, adrenocorticotropic hormone, and even cortisol) can quite rapidly convert V into T HFs (6) questions the non-convertibility hypothesis. The latter is based on unit area trichogram and phototrichogram, which relies on defining V HFs based on their visible hair shaft and thus has inherent methodological limitations (7).
MATERIALS AND METHODS
Quantitative histomorphometry of horizontally sectioned lesional mpAGA scalp skin biopsies are the most accurate method for assessing HF miniaturization and its reversal (3, 8, 9). Using this more accurate methodology, we asked in the current pilot studywhether a former T HF, which has been transformed into a V HF under androgen-stimulation, can be reconverted to a T HF under standard AGA therapy in a well-defined area of mpAGA scalp skin in vivo. To address this question, we have re-analysed human scalp skin samples derived from our novel humanized mpAGA mouse model, in which human mpAGA scalp xenotransplants had been treated long-term with MXL or platelet-rich plasma (PRP) in vivo (10, 11), applying the objective histomorphometry criteria listed in Fig. 1A.
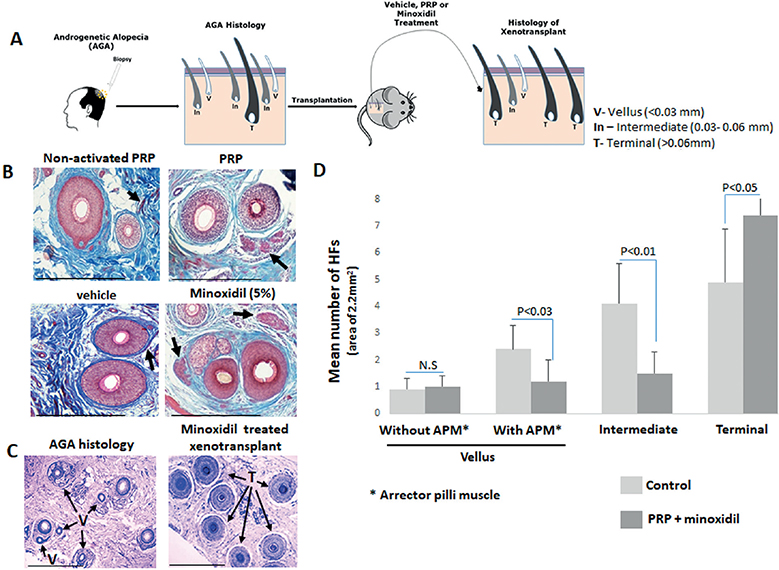
Fig. 1. Arrector pili muscle in male pattern androgenetic alopecia (mpAGA) xenotransplants treated with activated platelet-rich plasma (PRP) or minoxidil 5% (MXL) vs control. (A) Overview of the experimental design. Biopsies were obtained from 10 patients with androgenetic alopecia (AGA) and transplanted onto 19 severe combined immunodeficiency (SCID)/beige mice, which were treated with either MXL or PRP (10, 11). The paraffin-embedded sections were cut and stained with haematoxylin and eosin (H&E) to define the hair follicles (HFs) according to their hair shaft diameter (3) and with Mason’s trichrome to distinguish the red-staining arrector pili muscle (APM) (7). (B) Higher magnification of vellus (V) HFs with preserved APM in treated vs vehicle control xenotransplants. (C) Representative photomicrographs of horizontal sections shows a predominance of V and intermediate HFs in mpAGA skin before transplantation compared with a majority of terminal (T) HFs in xenotransplants treated for 4 months with topical MXL. (D) Quantitative histomorphometry demonstrates that the number of V HFs without APM remained almost constant in treated vs control xenotransplants, while a significantly decreased number of vellus with APM and of intermediate HFs was seen in treated vs control xenotransplants. In parallel, the number of T HFs in xenotransplants treated with PRP or MXL increased significantly compared with the control xenotransplants. Data are presented as the mean ± standard error of mean (SEM). Statistical significance was set at p-value < 0.05 and calculated by non-parametric Kruskal-Wallis test, followed by a Mann–Whitney U test. Scale bars: 50 µm.
A total of 57 lesional biopsies from 10 mpAGA patients (mean age 35.9 ± 9.4 years) obtained after informed patient consent and IRB approval, which had been transplanted onto SCID/beige mice (10, 11), were re-examined histologically. Five mice were treated once daily with 5% topical MXL, 4 mice with vehicle, 5 mice once monthly for 4 months with intradermal injection of non-activated autologous PRP (control) and 5 mice with activated autologous PRP, prepared and activated as described (10). Each mouse was transplanted with 3 xenotransplants. In our previous reports (10, 11), we had not interrogated therapy-induced changes in the % of V, ”intermediate”, and T HFs in the mpAGA-affected human scalp skin xenotransplants. Namely, we searched for post-therapy changes in the number of V HFs with an associated arrector pili muscle (APM), whose presence is thought to identify those V HFs that once had been a T HF (7), while HFs that always were V typically lack an APM (12).
Quantitative histomorphometry of the xenotransplants after 4 months of therapy with either MXL or PRP compared with vehicle or non-activated-PRP showed a significantly decreased number of V HFs with an associated APM (p <0.05), i.e. of those HFs that likely had undergone prior T→V conversion during mpAGA (7) (Fig. 1B and Tables SI and SII). Moreover, compared to vehicle-treated controls, the MXL- or PPR-treated mpAGA scalp skin xenotransplants also showed significantly fewer intermediate HFs (p <0.01) (Fig. 1C, D and Tables SI and SII). Intermediate HFs are overall shorter, and display smaller hair shaft, bulb, and dermal papilla diameter, and thus represent a T→V transition stage that precedes complete HF miniaturization (4, 13). Inversely, this was accompanied by a significant increase in the number of T HFs (p < 0.05) in MXL- or PPR-treated xenotransplants compared with vehicle or non-activated PRP (Fig. 1D).
The number of V without APM remained constant between the test and control xenotransplant groups (Fig. 1D). This is interesting in the context of Sinclair’s hypothesis (7) that loss of contact between the APM and the stem cell-rich bulge in AGA may render HF miniaturization irreversible (14) and might be interpreted as supporting this hypothesis. Alternatively, this could indicate that HFs in mature adult human scalp skin, which always represented the V phenotype, are much less responsive to MXL and PRP treatment than V HF that are miniaturized former T HFs.
DISCUSSION
These histomorphometric data demonstrate that, in line with Whiting (3), V→T reconversion of V HFs can indeed occur under therapy, even in long-standing mpAGA, at least in this humanized mouse model in vivo. Theoretically the reduced number of vellus HFs after therapy may also reflect deletion of vellus HFs, as HFs can undergo ”programmed organ deletion” (15), although no AGA therapy has been shown to promote this phenomenon.
Of course, one needs to consider that xenotransplantation is initially associated with a temporary wound-healing response and that male mouse testosterone serum levels tend to be substantially lower than those in human males, which might have impacted our results. However, murine dihydrotestosterone serum levels are actually quite similar to the human ones (16) which renders it unlikely that human scalp skin xenotransplants grow in a state of relative systemic hypoandrogenism thatmight have artificially facilitated V→T HF reconversion in our in vivo system. Moreover, potential HF reconversion effects of the initial wound-healing response post-transplantation would have affected both test and control xenotransplants and would hardly still be visible 4 months later.
Therefore, our pilot in vivo study questions the validity of the Rushton and Van Neste et al. (1) hypothesis by showing that standard clinical mpAGA therapy can promote a V→T reconversion in lesional human mpAGA skin in principle, at least in miniaturized V HFs that have not lost contact with their APM. While the current study data in a humanized mouse model of AGA obviously require biopsy- and histomorphometry-based confirmation in mpAGA patients under therapy, there is no biologically compelling reason why V→T HF reconversion should not also be possible under clinical conditions. As predicted from previous ex vivo work with organ-cultured human scalp HFs (4), the current study data also suggest that, unsurprisingly, intermediate HFs are more reconversion-responsive than fully miniaturized V mpAGA HFs (Fig. 1A, D and Tables SI and SII).
A long-term, large-scale, biopsy-based clinical study on patients with mpAGA, using the same definitive quantitative histomorphometry read-out parameters as employed here, is needed to confirm the findings of the current in vivo pilot study. It is possible that a larger clinical study could render non-surgical therapies in mpAGA management more promising than has recently been claimed (1), and make early therapeutic intervention strongly advisable.
ACKNOWLEDGEMENTS
This study was supported in part by the Technion Research and Development Foundation (TRDF) to A.G. and an Endowed Frost Scholarship to R.P.
REFERENCES
- Rushton DH, Westgate GE, Van Neste DJ. Following historical “tracks” of hair follicle miniaturisation in patterned hair loss: are elastin bodies the forgotten aetiology? Exp Dermatol 2022; 31: 102–109.
- Guarrera M, Rebora A. The higher number and longer duration of kenogen hairs are the main cause of the hair rarefaction in androgenetic alopecia. Skin Appendage Disord 2019; 5: 152–154.
- Whiting DA, Waldstreicher J, Sanchez M, Kaufman KD. Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men and postmenopausal women. J Investig Dermatol Symp Proc 1999; 4: 282–284.
- Miranda BH, Charlesworth MR, Tobin DJ, Sharpe DT, Randall VA. Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life. FASEB J 2018; 32: 795–806.
- Tsuboi R, Niiyama S, Irisawa R, Harada K, Nakazawa Y, Kishimoto J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: a randomized placebo-controlled double-blinded dose-finding clinical study. J Am Acad Dermatol 2020; 83: 109–116.
- Hawkshaw NJ, Paus R. Beyond the NFAT horizon: from cyclosporine A-induced adverse skin effects to novel therapeutics. Trends Pharmacol Sci 2021; 42: 316–328.
- Sinclair R. Androgenetic alopecia. Modelling progression and regrowth. Exp Dermatol 2016; 25: 424–425.
- Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al.; European Consensus Group. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 2011; 164: 5–15.
- Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ, et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol 2016; 136: 34–44.
- Laufer Britva R, Keren A, Ginzburg A, Ullmann Y, Paus R, Gilhar A. Evidence from a humanized mouse model of androgenetic alopecia that platelet-rich plasma stimulates hair regrowth, hair shaft diameter and vellus-to-terminal hair reconversion in vivo. Br J Dermatol 2021; 185: 644–646.
- Gilhar A, Keren A, Ullmann Y, Wu J, Paus R. Effect of minoxidil formulations on human scalp skin xenotransplants on SCID mice: a novel pre-clinical in vivo assay for androgenetic alopecia research. Exp Dermatol 2022; 31: 980–982.
- Blume U, Ferracin J, Verschoore M, Czernielewski JM, Schaefer H. Physiology of the vellus hair follicle: hair growth and sebum excretion. Br J Dermatol 1991; 124: 21–28.
- Piccini I, Sousa M, Altendorf S, Jimenez F, Rossi A, Funk W, et al. Intermediate hair follicles from patients with female pattern hair loss are associated with nutrient insufficiency and a quiescent metabolic phenotype. Nutrients 2022; 14: 3357.
- Yazdabadi A, Whiting D, Rufaut N, Sinclair R. Miniaturized hairs maintain contact with the arrector pili muscle in alopecia areata but not in androgenetic alopecia: a model for reversible miniaturization and potential for hair regrowth. Int J Trichology 2012; 4: 154–157.
- Eichmüller S, van der Veen C, Moll I, Hermes B, Hofmann U, Müller-Röver S, et al. Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem 1998; 46: 361–370.
- Fu D, Huang J, Li K, Chen Y, He Y, Sun Y, et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed Pharmacother 2021; 137: 111247.