ORIGINAL REPORT
Discontinuation of Fumaric Acid Esters is Affected by Depressive Symptomatology: A Retrospective Analysis
Frederik KREFTING1#, Stefanie HÖLSKEN2#, Manfred SCHEDLOWSKI2,3 and Wiebke SONDERMANN1
1Department of Dermatology, Venereology and Allergology and 2Institute of Medical Psychology and Behavioral Immunobiology, University Hospital Essen, University of Duisburg-Essen, Essen, Germany, and 3Department of Clinical Neuroscience, Osher Center for Integrative Medicine, Karolinska Institutet, Stockholm, Sweden
#These authors contributed equally.
Fumaric acid esters (FAEs) remain a widespread therapy option for moderate-to-severe psoriasis. However, drug survival of FAEs is limited by adverse events (AEs) or inadequate treatment response. Depressive disturbances are highly prevalent in psoriasis patients and are hypothesized to be associated with the reporting of AEs and therapy discontinuation. This study’s aim was to analyze whether psoriasis patients with comorbid depressive symptomatology are more likely to discontinue treatment with FAEs due to AEs and/or inadequate treatment response. Data were retrospectively extracted from the records of patients starting therapy with FAEs in the Department of Dermatology, University Hospital Essen, Germany between 2017 and 2022, covering the first 52 weeks of treatment. Psoriasis severity and depressive symptomatology, as well as AEs and therapy discontinuation, were analyzed. Psoriasis patients (N = 95, 47.37% female) with depressive symptomatology (42.11%) were more likely to discontinue therapy due to patient-reported AEs, while the total number of reported AEs was not associated with depression. The results support the hypothesis that among psoriasis patients with depressive symptoms, the associated introspection and somatization may result in increased sensitivity for AEs and thus in quicker therapy discontinuation. In these patients, the occurrence of nocebo effects should be minimized, e.g. by special communication techniques.
SIGNIFICANCE
Fumaric acid esters are an established systemic treatment option for psoriasis patients. However, unwanted side effects frequently lead to premature therapy discontinuation. Thus, we investigated whether psoriasis patients with depressive symptomatology – a common comorbidity of psoriasis – are more likely to discontinue therapy with fumaric acid esters compared with patients without evidence of depression. We found that depressive patients showed a higher probability of discontinuing treatment because of subjectively experienced treatment side effects, which could be due to increased introspection and somatization associated with depression. Future studies should aim at employing interventions to avoid premature therapy discontinuation in these patients.
Key words: adverse events; depression; discontinuation; fumaric acid esters; psoriasis.
Citation: Acta Derm Venereol 2024; 104: 12326. DOI: https://doi.org/10.2340/actadv.v104.12326.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Apr 13, 2023. Accepted after review: Feb 22, 2024. Published: Mar 14, 2024.
Corr: Wiebke Sondermann, MD, Department of Dermatology, Venereology and Allergology, University Hospital Essen, University of Duisburg-Essen, Hufelandstr. 55, DE-45122 Essen, Germany. E-mail: wiebke. sondermann@uk-essen.de
Competing interests and funding: FK Krefting has received travel support and/or consulting fees from Almirall and Novartis outside the submitted work. WS reports grants from Almirall with reference to the submitted work and/or travel support and/or personal fees and/or speaker honoraria from medi GmbH Bayreuth, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, GSK, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi Genzyme, and UCB outside the submitted work. MS reports speaker honoraria from Almirall, Lilly, Bristol-Myers Squibb, Janssen, Berlin Chemie, Abbvie, and Mylan outside the submitted work. SH declares no conflicts of interest.
INTRODUCTION
Almost 3% of the total western population suffers from psoriasis, a chronic inflammatory disease associated with typical erythematosquamous skin plaques (1). In parallel to the increasing scientific understanding of psoriasis as a systemic inflammatory disease, various comorbidities are known to occur in addition to the classic skin symptoms (2). For instance, psoriatic arthritis is reported to be present in about 30% of psoriasis patients. Further, several epidemiological studies demonstrated an increased prevalence of cardiovascular and cardiometabolic risk factors such as arterial hypertension (3), obesity (4, 5), insulin resistance (6), or diabetes mellitus (7). Psychological comorbidities like depression and anxiety, as well as suicidal ideation and behavior, are also frequently associated with psoriasis (8–11).
Fortunately, many different systemic therapy options are available nowadays to treat psoriasis (12, 13). Modern biologics have been shown to be highly effective; however, these medications are expensive and can place a high financial burden on the healthcare system, if frequently and irrationally prescribed (14, 15). Fumaric acid esters (FAEs) remain an appropriate and much more cost-effective therapy option for patients with moderate to severe psoriasis (12). For example, the annual therapy costs of FAEs such as Skilarence® amount to about €5,800 per patient in Germany and are thus significantly lower than those of biologics, which can amount to up to more than €20,000 annually (16).
However, the occurrence of adverse events (AEs) such as gastrointestinal side effects or flushing symptoms, and also an inadequate response to treatment with FAEs, can lead to discontinuation of therapy in a significant proportion of patients. In the “BRIDGE” study, which paved the way for the approval of FAEs in Europe, approximately 23% of patients receiving FAEs discontinued treatment due to AEs within the 16-week follow-up time of the study (17). Real-world data from Ireland showed a 4-year drug survival of FAEs of 60% (18). In this study, 40% of all patients analyzed terminated therapy due to a lack of efficacy and 47% because of side effects such as gastrointestinal symptoms (27%). Treatment discontinuation was most common in the first year after therapy initiation (18). More recent data showed somewhat increased rates of discontinuation, with drug survival ranging from 51% to 55% (19, 20).
As these frequent terminations of therapy with FAEs pose a burden both for patients and for the health system, evaluation of the risk factors and reasons for therapy terminations is needed.
An established reason for the occurrence of side effects in many therapies are so-called nocebo effects. The term “nocebo effect” generally describes the appearance of AEs that cannot be traced back to an administered drug – a frequent observation in the placebo arms of clinical trials (21). In particular, patients with psychiatric disorders such as depression or anxiety – both frequent comorbidities of psoriasis – have been shown to display an increased risk of nocebo effects (8–11, 22). These nocebo effects may lead to an increased perception of especially subjective therapeutic side effects and faster therapy termination by patients (23). Apart from not recommending systemic treatments with brodalumab and apremilast, neither the German nor the European guideline favors any specific systemic therapy for psoriasis patients with depression (24, 25). In addition, data suggest that women may be more susceptible to nocebo effects and less tolerant of side effects (22, 26).
Based on the assumption that a depressive symptomatology may foster nocebo effects, this study aimed to investigate to what extent psoriasis patients with a comorbid depressive symptomatology were more likely to discontinue treatment with FAEs due to inadequate treatment response and/or due to occurrence of AEs.
MATERIALS AND METHODS
Ethics
The study was approved by the institutional ethics committee of the University of Duisburg-Essen (21-10348-BO) and conducted in accordance with the Declaration of Helsinki.
Data collection
Data were extracted from the records of patients starting therapy with FAEs at the Department of Dermatology, University Hospital Essen, Germany between April 2017 and March 2022. Relevant data included demographic information, information on the existence of a comorbid depression, and information concerning the patients’ psoriasis severity at the beginning and end of therapy, the occurrence of AEs, and the circumstances of possible therapy discontinuation. The observed period for each patient included the first 52 weeks of treatment, as previous studies could show that the majority of treatment discontinuations occurred within this time frame (18).
Within the clinical routine, depressive symptoms were assessed using either the Two Questions Test (TQT [27–29]) or the Beck Depression Inventory II (BDI [30]). In the TQT, which is also known as the Whooley Questions, patients need to answer the following questions with yes or no: “Have you frequently felt dejected, sad, glum or hopeless during the past month?” and “Have you taken significantly less pleasure and joy in things, which you otherwise enjoy doing, over the past month?”. The test was found to possess a high sensitivity of 95% and a moderate specificity of 65% (31). The BDI consists of 21 questions with four possible answers and is an established tool with good psychometric properties (32, 33). We assumed a depressive symptomatology (for reasons of simplicity hereafter termed “depression”) if at least one of the following criteria was present: (i) depression recorded as a comorbidity, (ii) a score of at least one point on the TQT, or (iii) a score of at least 11 points on the BDI.
The severity of skin lesions was documented using the Psoriasis Area and Severity Index (PASI [34]). Potential AEs of therapy were labeled as “reported” when stated by the patients or “observed” when detected in the patients’ blood work.
Data analysis
Data was compiled using IBM SPSS Statistics (version 27; IBM Corp, Armonk, NY, USA) and analyzed in R (version 4 [35]). Power analyses were performed using G*Power (version 3.1) (36). Figures were created using the R package ggplot2 (37).
The association between “depression” and sex on the one hand and treatment discontinuation on the other hand was analyzed employing logistic regression. Discontinuation of therapy due to inadequate response was objectified by non-achievement of a PASI75 response (12).
To better illustrate the interaction of treatment discontinuation and depressive symptomatology, descriptive data were presented for three different levels of depression severity: a “low depression threshold” group included all patients meeting the following criteria: (i) depression recorded as a comorbidity, or (ii) at least 1 point on the TQT, or (iii) at least 11 points on the BDI. “High depression threshold” included patients for whom at least 1 of the following criteria was met: (i) depression recorded as a comorbidity, (ii) a score of 2 points on the TQT, or (iii) a score of at least 18 points on the BDI. A third group called “diagnosed depression” consisted only of those patients for whom a depression was registered as a comorbidity. All patients who met none of the criteria for “low depression threshold” were included in a “no evidence of depression” group.
RESULTS
Sample
In total, 98 patients who started treatment with FAEs were identified. All patients received FAEs as a first-line systemic therapy and none suffered from psoriatic arthritis. N = 3 patients had to be excluded from the analysis, as their files were incomplete, resulting in a final sample of N = 95 patients (47.37% female). Sample characteristics are illustrated in Table I. Information on baseline depression status was available for all included patients. At baseline, 10.53% of patients presented with a diagnosis of comorbid depression. The criterion of “depression” as defined earlier was met by 42.11% of patients. At baseline, seven patients were taking antidepressants, including venlafaxine (N = 2), sertraline (N = 2), fluoxetine (N = 1), agomelatine (N = 1), and citalopram (N = 1). An overview of the other comorbidities of the patients can be found in Table II.
| All patients n = 95 | No evidence of depressiona n = 55 | Low depression thresholdb n = 40 | High depression thresholdc n = 35 | Diagnosed depression n = 10 | |
| Female, % | 47.37 | 36.36 | 62.50 | 68.57 | 70.00 |
| Age, years, median (range) | 47.86 (16.72–80.19) | 51.62 (16.72–78.20) | 36.16 (18.02–80.19) | 36.70 (18.02–80.19) | 40.63 (21.32–75.27) |
| Disease duration of psoriasis, years, median (range) | 6.00 (0.00–56.00) | 5.50 (0.00–56.00) | 7.00 (0.00–26.00) | 6.00 (0.00–26.00) | 3.00 (0.0–26.00) |
| PASI, median (range) | 8.30 (1.20–22.70) | 8.82 (1.20–22.00) | 8.00 (1.50–22.70) | 7.80 (1.50–22.70) | 5.85 (2.00–22.70) |
| DLQI, median (range) | 14.00 (1.00–29.00) | 11.00 (1.00–26.00) | 16.95 (3.00–29.00) | 18.00 (4.00–29.00) | 18.67 (4.00–29.00) |
| aPatients with 0 points on the Two Questions Test (TQT), < 11 points on the Beck Depression Inventory (BDI), and no diagnosis of depression. bPatients with at least 1 point on the TQT, 11 points on the BDI, or diagnosed depression. cPatients with 2 points on the TQT, 18 points on the BDI, or diagnosed depression. PASI: Psoriasis Area and Severity Index; DLQI: Dermatological Life Quality Index; n: number of patients on whom this information was provided in the files. |
|||||
Description of treatment discontinuation
N = 60 (63.16%) patients discontinued treatment within the first 52 weeks after initiation. The most common reason for treatment discontinuation was the occurrence of AEs (70.00%), followed by inadequate treatment response (15.00%). In 8.33% of patients, a combination of both AEs and an inadequate response led to discontinuation of FAEs and for 6.67% there were other reasons (such as a planned pregnancy). Time until treatment discontinuation was shortest for those patients who discontinued due to AEs. There was no influence of “depression” or sex on the chances of discontinuing treatment when analyzed independently of the reasons (results in Table III).
Analysis of treatment discontinuation due to inadequate response
Of those patients discontinuing treatment due to inadequate treatment response, none had reached a PASI75 reduction at discontinuation. Thus, we can assume that all discontinuing patients showed an objectively inadequate treatment response. The influence of the predictors “depression” and sex on the discontinuation due to inadequate response was analyzed using logistic regression. There was neither a significant effect of “depression” (z = 0.13, n.s,) or sex (z = –1.32, n.s.), nor a significant interaction effect of the two factors (z = 0.25, n.s.). The model effect size expressed as Nagelkerke’s R2 (R2N) was 0.043, indicating that 4.3% of the variance in therapy discontinuation was explained by the model. Descriptively, the odds of treatment discontinuation were slightly increased for patients with “depression” (OR = 1.09, 95% CI [0.28, 3.89], power = 0.05) and decreased for women (OR = 0.38, 95% CI [0.07, 1.46], power = 0.45) (Fig. 1).
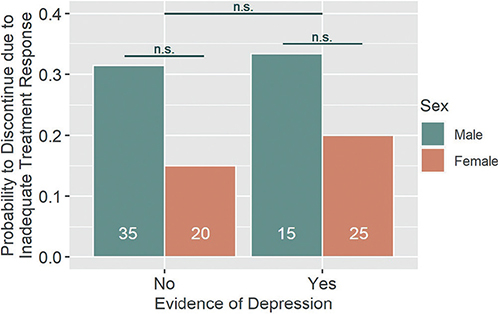
Fig. 1. Discontinuation due to inadequate treatment response. Patients with or without evidence of depression as well as male and female patients did not statistically differ with regard to their likelihood to discontinue treatment due to inadequate treatment response. There was no significant interaction effect of the two factors. n.s.: not statistically significant. White numbers within columns represent the sample size of each group.
Analysis of treatment discontinuation due to observed adverse events
The two factors “depression” and sex could not significantly predict treatment discontinuation due to AEs observed in the patients’ blood work, with z = –1.43 (n.s.) for “depression”, z = –0.48 (n.s.) for sex, and z = 0.97 (n.s.) for their interaction effect. The model effect size was R2N = 0.050. Here, the descriptive results indicate slightly decreased odds of treatment discontinuation in patients with “depression” (OR = 0.21, 95% CI [0.01, 1.27], power = 0.63) as well as in women (OR = 0.72, 95% CI [0.17, 2.63], power = 0.10) (Fig. 2).
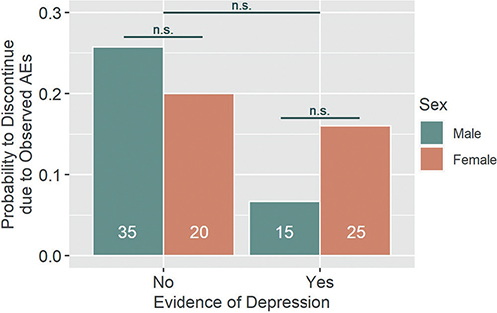
Fig. 2. Discontinuation due to observed adverse event (AEs). Patients with or without evidence of depression as well as male and female patients did not statistically differ with regard to their likelihood to discontinue treatment due to clinician-observed AEs. There was no significant interaction effect of the two factors. n.s.: not statistically significant. White numbers within columns represent the sample size of each group.
Analysis of treatment discontinuation due to reported adverse events
The most commonly reported AEs were gastrointestinal symptoms (N = 61). A full list can be found in Table IV. There was a statistically significant effect of “depression” on the discontinuation due to reported AEs (z = 2.28, p = 0.023), with patients suffering from ”depression“ showing increased odds of treatment discontinuation (OR = 4.57, 95% CI [1.26, 17.78], power = 0.92). Sex did not significantly affect treatment discontinuation (z = 1.22, n.s.) and there was no significant interaction effect (z = –1.08, n.s.). Descriptively, the odds of discontinuation were increased in women (OR = 2.15, 95% CI [0.62, 7.59], power = 0.37). The model effect size was R2N = 0.106. For a visualization, see Fig. 3.
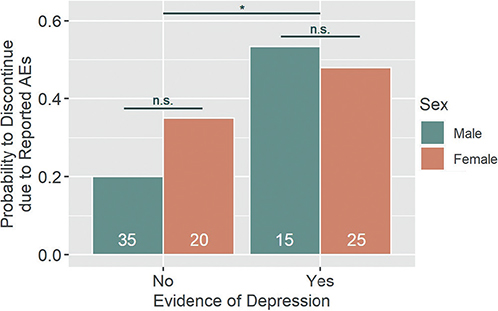
Fig. 3. Discontinuation due to reported adverse event (AEs). Patients showing evidence of depression were significantly more likely to discontinue treatment due to patient-reported AEs. Patients’ sex did not influence this likelihood, nor was there a significant interaction effect of the two factors. n.s.: not statistically significant. *p-value < 0.05. White numbers within columns represent the sample size of each group.
In an additional logistic regression analysis, the effect of “depression” and sex on the actual occurrence of reported AEs was analyzed. There was no significant association between AEs and “depression” or between sex and AEs (z = 1.17, n.s.; z = 0.92, n.s., respectively). The interaction effect of “depression” and sex was also not statistically significant (z = 0.90, n.s.). On a descriptive level, there was an increase in the odds of reported AEs in both patients suffering from “depression” (OR = 2.36, 95% CI [0.61, 11.80]) and women (OR = 1.77, 95% CI [0.54, 6.48]) with an effect size of R2N = 0.162.
To illustrate the effect of depressive symptoms on treatment discontinuation due to reported AEs, the probability of discontinuation was calculated for five different groups as illustrated in Fig. 4. This analysis found an increased probability of discontinuation in the more severe cases of depressive symptomatology.
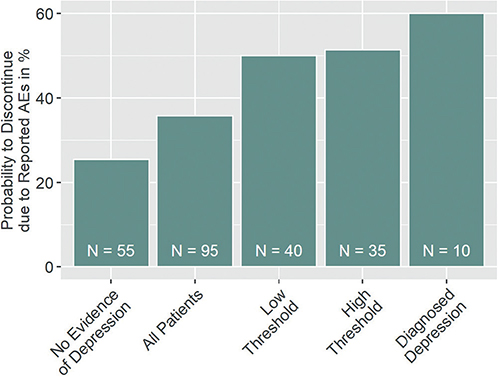
Fig. 4. Probability of treatment discontinuation due to reported adverse event (AEs) by depression status. The probability of treatment discontinuation due to reported adverse events AEs increases with the level of evidence for patients’ depression. No evidence of depression: patients with 0 points on the Two Questions Test (TQT), < 11 points on the Beck Depression Inventory (BDI), and no diagnosis of depression; low threshold = patients with at least 1 point on the TQT, 11 points on the BDI, or diagnosed depression; high threshold = patients with 2 points on the TQT, 18 points on the BDI, or diagnosed depression.
DISCUSSION
The aim of the present work was to analyze a possible association between the presence of increased depressive symptomatology and an elevated rate of discontinuation of therapy with FAEs due to side effects possibly mediated by nocebo effects. With this aim, data from patient records were analyzed retrospectively, taking into account a possible termination of FAE therapy and the reasons for termination, as well as patients’ depressive symptoms and clinician-assessed treatment success.
Following the initial hypothesis, our data indicate that patients with depression are more likely to discontinue therapy due to reported AEs such as gastrointestinal symptoms or flushing. Therapy discontinuation following clinician-observed AEs in the patients’ blood work or discontinuation due to inadequate treatment response on the other hand were not affected by patients’ depressive symptomatology. The observed effect could either result from an increment in the occurrence of reported AEs in depressed patients, or, alternatively, from an increase in therapy discontinuations despite similar rates of AEs. Our analysis could not find any evidence of an increment in reported symptoms in said patients, suggesting that the mechanism might actually be that of perception and tolerance of AEs. On the one hand, patients with depression tend towards intensified introspection and somatization (38), which could affect the perception of AEs. On the other hand, depression is a well-known risk factor for non-adherence. For example, one meta-analysis found a threefold increased risk of non-adherence in the cohort analyzed (39). In the case of our sample, it is conceivable that the combination of mental distress in addition to the experience of AEs might be too much to bear, leading to quicker therapy discontinuations.
Depressive symptoms themselves might also lead to therapy recommendations being harder to implement, resulting in less competent coping with and tolerance of AEs. However, as no comparable studies have been published so far, the question whether the rate of reported symptoms themselves or only the rate of resulting discontinuations is increased in this patient cohort needs further validation in future studies. With a rate of treatment discontinuation of over 60%, the drug survival in this study is significantly worse than in the real-world data from Ireland presented earlier (18). However, more recent studies report a generally decreased drug survival of 51% and 55% (19, 20). This decrease may be the result of adjusted therapy recommendations, which make the prescription of a biologic easier for clinicians (12). In addition, the Department of Dermatology at the University Hospital Essen is a tertiary specialist center for the treatment of psoriasis, which may be associated with an accumulation of complex cases, contributing to even worse drug survival.
As the presented data are based on a retrospective analysis of patient records, our data have certain limitations. On the one hand, we do not know how the medication was initially presented – FAEs may have been presented as a “first try” before the prescription of a biologic, resulting in dampened expectations by patients, which may in turn lead to reduced therapy success (40). The same is true for the communication with regard to AEs: these may have been presented as likely to occur, fostering the occurrence of nocebo effects (41). On the other hand, we have to rely on the patients’ report when it comes to AEs such as gastrointestinal symptoms. Some patients may have known that they could receive more effective medication when presenting with such symptoms. Another limitation of the study may be that the level of anxiety in patients, which is associated with nocebo effects (42), was not assessed in our study cohort but should be considered in follow-up studies. At the same time, the reliance on real-world data is a strength of this study: rather than in a study setting, data were collected within the clinical routine, providing a maximum of external validity. Lastly, even though our clinic is a large center for psoriasis treatment, the number of patients having received FAEs whose data could be used for this study is limited. Sample size was sufficient to assess the main question, but with regard to the stratified analyses of sex, power was insufficient to determine statistically significant differences. We hope that this study can therefore serve as a pilot to encourage similar assessments in larger cohorts, to validate the seen effects.
The presented data indicate an increased vulnerability for the discontinuation of FAE therapy due to reported AEs in patients with evidence of depression. This might have important implications for clinical practice. Psoriasis patients should be screened for the presence of depression as part of the clinical routine via their patient history and/or questionnaires, such as the TQT (27–29) or the BDI (30). If a depressive symptomatology is indicated, special attention to the prevention of AEs may be helpful in consultation with these patients, as there are several communication techniques that can help to minimize the occurrence of nocebo effects (43). Clinicians could also offer low-threshold support (i.e. a direct dial number) in the event that AEs occur. Furthermore, psychotherapeutic support to build up resiliency may help these patients to cope with AEs. In conclusion, the results of this study suggest that while the total rate of reported AEs is not increased in patients with depressive symptomatology, this patient group still shows a higher probability of discontinuing treatment due to the experience of AEs. Future studies should aim at unravelling the mechanisms underlying this increased vulnerability as well as employing interventions to support this patient cohort.
ACKNOWLEDGEMENTS
The authors would like to thank Lea Schneider for supporting this work. The data that support this work will be made available at the Center for Open Science (https://www.osf.io). The abstract of this paper has been accepted for publication at the 4th International Conference of the Society for Interdisciplinary Placebo Studies (SIPS).
The study was supported by Almirall. There was no involvement of the funding body in writing this manuscript.
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 422744262 – TRR 289).
The study was approved by the institutional ethics committee of the University Duisburg-Essen (21-10348-BO) and conducted in accordance with the Declaration of Helsinki.
REFERENCES
- Schäfer I, Rustenbach SJ, Radtke M, Augustin J, Glaeske G, Augustin M. Epidemiologie der Psoriasis in Deutschland – Auswertung von Sekundärdaten einer gesetzlichen Krankenversicherung. Gesundheitswesen 2011; 73: 308–313.
- Dauden E, Blasco AJ, Bonanad C, Botella R, Carrascosa JM, Gonzalez-Parra E, et al. Position statement for the management of comorbidities in psoriasis. J Eur Acad Dermatol Venereol 2018; 32: 2058–2073.
- Armesto S, Coto-Segura P, Osuna CG, Camblor PM, Santos-Juanes J. Psoriasis and hypertension: a case-control study. J Eur Acad Dermatol Venereol 2012; 26: 785–788.
- Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med 2007; 167: 1670–1675.
- Correia B, Torres T. Obesity: a key component of psoriasis. Acta Biomed 2015; 86: 121–129.
- Gyldenlove M, Storgaard H, Holst JJ, Vilsboll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol 2015; 72: 599–605.
- Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol 2016; 152: 761–767.
- Hölsken S, Krefting F, Schedlowski M, Sondermann W. Common fundamentals of psoriasis and depression. Acta Derm Venereol 2021; 101: adv00609.
- Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol 2017; 31: 1999–2009.
- Tribo MJ, Turroja M, Castano-Vinyals G, Bulbena A, Ros E, Garcia-Martinez P, et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Derm Venereol 2019; 99: 417–422.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010; 146: 891–895.
- Nast A, Altenburg A, Augustin M, Boehncke WH, Härle P, Klaus J, et al. German S3-Guideline on the treatment of Psoriasis vulgaris, adapted from EuroGuiDerm – Part 1: Treatment goals and treatment recommendations. J Dtsch Dermatol Ges 2021; 19: 934–150.
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 2020; 34: 2461–2498.
- Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci 2020; 21: 1690.
- Hur P, Kim N, Dai D, Piao OW, Zheng JZ, Yi E. Healthcare cost and utilization associated with biologic treatment patterns among patients with psoriatic arthritis: analyses from a large US claims database. Drugs Real World Outcomes 2021; 8: 29–38.
- Gemeinsame Arbeitsgruppe Arzneimittel KVN, Verbände der gesetzlichen Krankenkassen in Niedersachsen. Medikamentöse Therapie bei Psoriasis vulgaris, 2022.
- Mrowietz U, Szepietowski JC, Loewe R, van de Kerkhof P, Lamarca R, Ocker WG, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm((R)) – and placebo-controlled trial (BRIDGE). Br J Dermatol 2017; 176: 615–623.
- Ismail N, Collins P, Rogers S, Kirby B, Lally A. Drug survival of fumaric acid esters for psoriasis: a retrospective study. Br J Dermatol 2014; 171: 397–402.
- Corazza M, Odorici G, Conti A, Di Lernia V, Motolese A, Bardazzi F, et al. Dimethyl fumarate treatment for psoriasis in a real-life setting: a multicentric retrospective study. Dermatol Ther 2021; 34: e15066.
- Pezzolo E, Cazzaniga S, Di Leo S, Naldi L, PsoReal Study G. Efficacy and safety of Dimethyl fumarate in comparison with conventional therapy for psoriasis: an Italian real-world clinical experience. J Eur Acad Dermatol Venereol 2022; 36: e534–e537.
- Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev 2015; 67: 697–730.
- Symon A, Williams B, Adelasoye QA, Cheyne H. Nocebo and the potential harm of ‘high risk’ labelling: a scoping review. J Adv Nurs 2015; 71: 1518–1529.
- Nestoriuc Y, von Blanckenburg P, Schuricht F, Barsky AJ, Hadji P, Albert US, et al. Is it best to expect the worst? Influence of patients’ side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol 2016; 27: 1909–1915.
- Nast A, Altenburg A, Augustin M, Boehncke WH, Harle P, Klaus J, et al. Deutsche S3-Leitlinie zur Therapie der Psoriasis vulgaris, adaptiert von EuroGuiDerm – Teil 2: Therapiemonitoring, besondere klinische Situationen und Komorbiditat. J Dtsch Dermatol Ges 2021; 19: 1092–1117.
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol 2021; 35: 281–317.
- Torres T, Puig L, Vender R, Lynde C, Piaserico S, Carrascosa JM, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol 2021; 22: 567–579.
- Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12: 439–445.
- Radtke MA, Mrowietz U, Feuerhahn J, Harter M, von Kiedrowski R, Nast A, et al. Early detection of comorbidity in psoriasis: recommendations of the National Conference on Healthcare in Psoriasis. J Dtsch Dermatol Ges 2015; 13: 674–690.
- Hölsken S, Krefting F, Schneider L, Benson S, Schedlowski M, Sondermann W. A brief screening tool for depression in psoriasis patients: the Two Questions Test in clinical practice. J Dermatol 2022; 49: 341–348.
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 1984; 40: 1365–1367.
- Bosanquet K, Bailey D, Gilbody S, Harden M, Manea L, Nutbrown S, et al. Diagnostic accuracy of the Whooley questions for the identification of depression: a diagnostic meta-analysis. BMJ Open 2015; 5: e008913.
- von Glischinski M, von Brachel R, Hirschfeld G. How depressed is “depressed”? A systematic review and diagnostic meta-analysis of optimal cut points for the Beck Depression Inventory revised (BDI-II). Qual Life Res 2019; 28: 1111–1118.
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8: 77–100.
- Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244.
- Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2022.
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191.
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag, 2009.
- Van Gundy K, Schieman S. Looking inward: introspectiveness, physical disability, and depression across the life course. Int J Aging Hum Dev 2001; 53: 293–310.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160: 2101–2107.
- Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 2013; 12: 191–204.
- Colloca L, Finniss D. Nocebo effects, patient–clinician communication, and therapeutic outcomes. JAMA 2012; 307: 567–568.
- Corsi N, Colloca L. Placebo and nocebo effects: the advantage of measuring expectations and psychological factors. Front Psychol 2017; 8: 308.
- Sondermann W, Reinboldt-Jockenhöfer F, Dissemond J, Pfaar O, Bingel U, Schedlowski M. Effects of patients’ expectation in dermatology: evidence from experimental and clinical placebo studies and implications for dermatologic practice and research. Dermatology 2021; 237: 857–871.